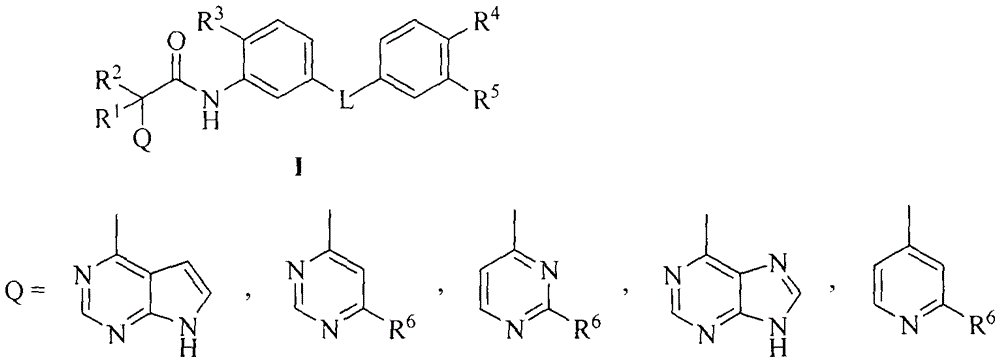
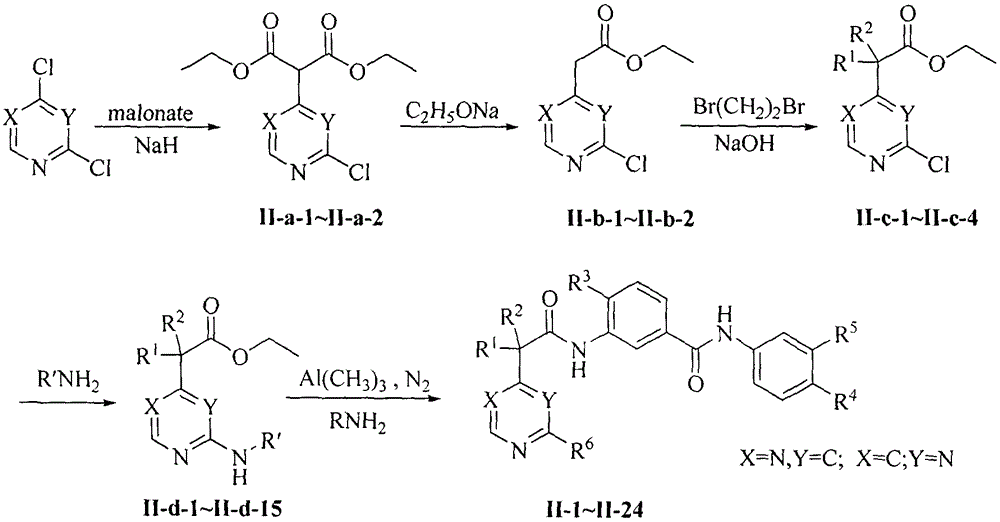
Novel aromatic amide Raf kinase inhibitors and preparation method and application thereof
A technology of benzamide and formamido, applied in the field of Raf kinase inhibitor, can solve the problems of unstable interaction, activation of downstream signals and uncontrolled cell growth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
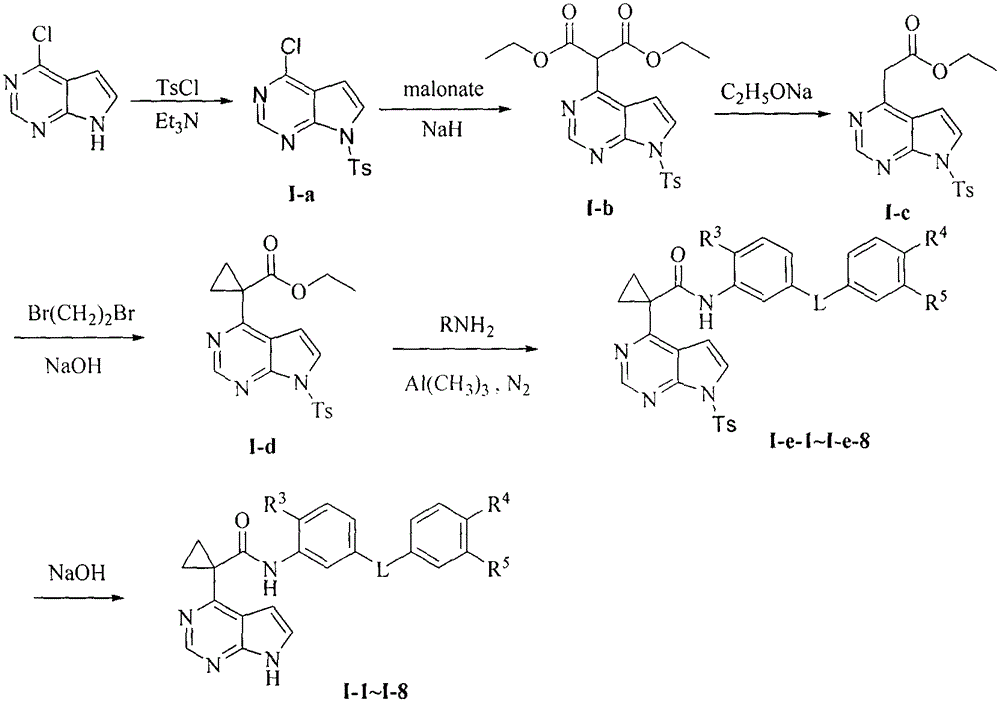
[0125] 4-Chloro-7-(4-methylbenzenesulfonyl)-7H-pyrrolo[2,3-d]pyrimidine (I-a)
[0126] Add 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (3.0g, 20mmo]) and triethylamine (6.1g, 60mmol) into a 250mL eggplant-shaped flask, add 100mL of anhydrous dichloromethane and stir to dissolve , added p-toluenesulfonyl chloride (2.6g, 24mmol), stirred at room temperature for 5h, and TLC detected that the starting point disappeared. The solvent was distilled off under reduced pressure, separated and purified by column chromatography to obtain 4.85 g of white solid I-a, and the yield was 79.2%. ESI-MSm / z: 308[M+H] + . 1 H-NMR (300MHz, DMSO-d 6 ): δ2.37 (3H, s, CH 3 ), 6.96 (1H, d, ArH, J=4.0Hz), 7.48 (2H, d, ArH, J=8.1Hz), 8.05 (2H, d, ArH, J=8.1Hz), 8.12 (1H, d, ArH, J = 4.0 Hz), 8.82 (1H, s, ArH).
Embodiment 2
[0128] Diethyl 2-[7-(4-methylbenzenesulfonyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]malonate (I-b)
[0129] Diethyl malonate (5.3g, 33mmol) was added to a 250mL eggplant-shaped flask, dissolved in 50mL of anhydrous THF, sodium hydride (60%, 800mg, 33mmol) was slowly added under ice-cooling and stirred for 5min, and I-a (1.0g , 3.3mmol) was dissolved in 20mL of anhydrous THF and slowly added to the reaction flask, refluxed for 2.5h, and TLC detected that the raw material point disappeared. Cool to room temperature, pour into 100 mL of saturated ammonium chloride solution, extract with ethyl acetate (50 mL×3), combine the organic layers, wash with saturated sodium chloride (30 mL×3), and dry over anhydrous magnesium sulfate overnight. The solvent was distilled off under reduced pressure and purified by column chromatography to obtain 1.4 g of a colorless oily substance I-b with a yield of 98.6%. ESI-MSm / z: 432[M+H] + .
Embodiment 3
[0131] 2-[7-(4-Methylbenzenesulfonyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]ethyl acetate (I-c)
[0132] Dissolve I-b (850mg, 2mmol) in 50mL of absolute ethanol, add sodium ethoxide (13mg, 0.2mmol), reflux for 2.5h, TLC detects that the raw material point disappears. Cool to room temperature, adjust the pH to neutral with dilute hydrochloric acid (1 mol / L), evaporate the solvent under reduced pressure, separate and purify by column chromatography to obtain 530 mg of white solid I-c, yield 77.0%. ESI-MSm / z: 360[M+H] + . 1 H-NMR (300MHz, DMSO-d 6 ): δ1.19 (3H, t, CH 2 C H 3 , J=7.1Hz), 2.36 (3H, s, ArCH 3 ), 4.08 (2H,q,C H 2 CH 3 , J=7.1Hz), 4.34 (2H, s, CH 2 CO), 7.03 (1H, d, ArH, J=4.0Hz), 7.46 (1H, d, ArH, J=8.2Hz), 7.99 (1H, d, ArH, J=4.0Hz), 8.04 (2H, d , ArH, J=8.2 Hz), 8.87 (1H, s, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com