Heterologous fusion gene modified cancer cell/dendritic cell fusion tumor vaccine and preparation method thereof
A technology that fuses cells and genes, applied in botany equipment and methods, biochemical equipment and methods, gene therapy, etc., can solve problems such as unsatisfactory therapeutic effects of fusion vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Example 1. Preparation of Fusion Cells
[0177] Preparation methods of fusion cells, including S1) and S2);
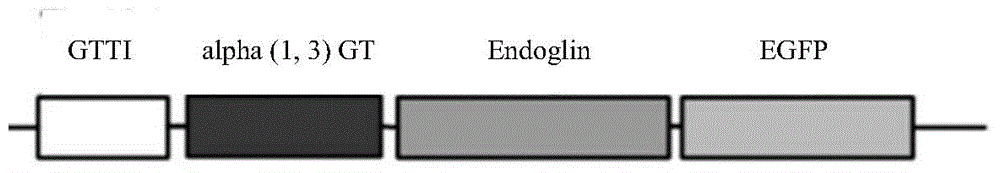
[0178] S1) Introduce the coding gene of transforming growth factor-β receptor related protein and α1,3-galactosyltransferase related gene into tumor cells to obtain recombinant cells;
[0179] S2) Fusion of the recombinant cell and dendritic cell to obtain the fused cell.
[0180] The specific method is as follows:
[0181] 1. Preparation of recombinant cells
[0182] 1. Preparation of recombinant cells
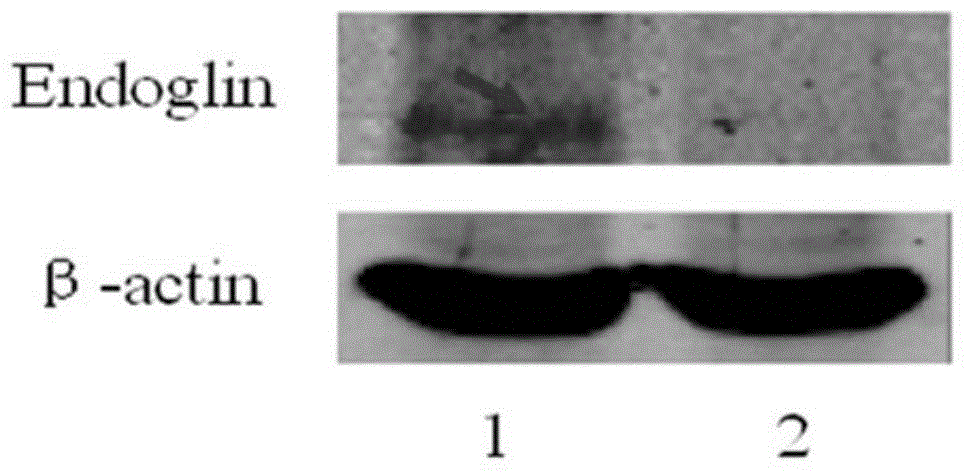
[0183] Replace the fragment between the BamHI and XbaI recognition sites on the pLVX-Puro vector with the DNA molecule shown in SEQ ID No. 5 in the sequence list, and keep the other sequences unchanged, to obtain the recombinant vector pLVX-Puro / GT-Eng, the recombinant vector pLVX -Puro / GT-Eng expresses the Endoglin protein shown in SEQIDNo.1 and the α1,3 galactosyltransferase (alpha(1,3)Galactosyltransferase, alpha(1,3)GT) shown in SEQIDNo.3. The recombinant vector pLVX...
Embodiment 2
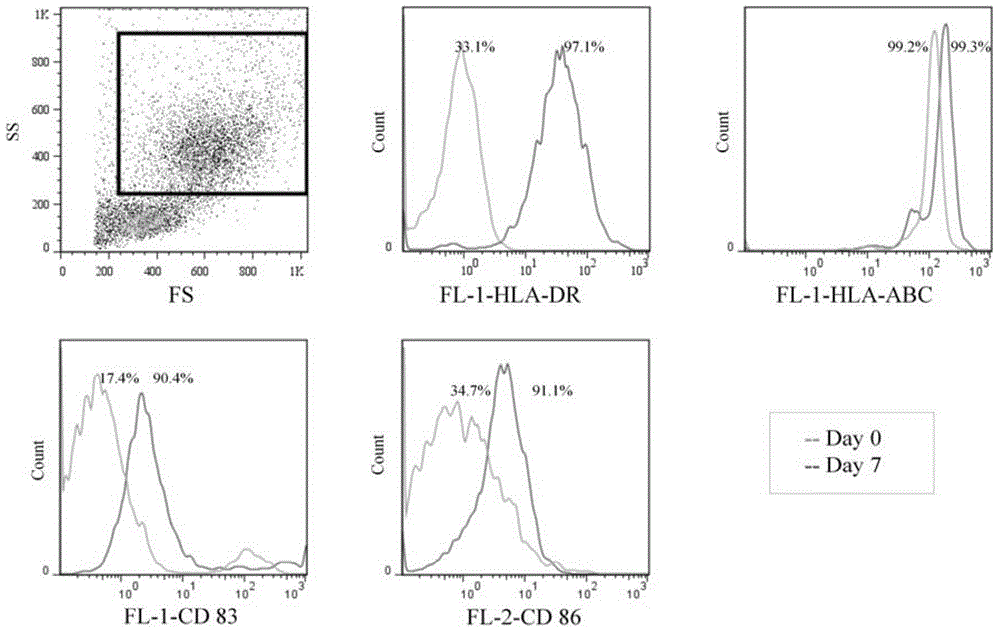
[0218] Example 2: DC / HepG2 (GT-Eng+) induces the production of IFN-γ-secreting T lymphocytes in vitro
[0219] The experiment was repeated three times, and the specific steps for each repeated experiment were as follows:
[0220] T lymphocytes secreting IFN-γ induced by DC / HepG2 (GT+) were obtained according to the following method, and the IFN-γ secreted by T lymphocytes was detected by enzyme-linked immunospot assay (ELISPOT). The 1×Washing buffer, biological The labeled antibody and enzyme-labeled avidin are all reagents in HumanIFN-gammaprecoatedELISPOTkit (HumanIFN-gammaprecoatedELISPOTkit is a product of Daktronics Biotechnology Co., Ltd., the catalog number is DKW22-1000-048). The specific steps are as follows:
[0221] 1) Take out the well plate in the kit, add 200 μL of incomplete RIPM1640 medium to each well, let it stand at room temperature for 5-10 minutes, and pour out the liquid.
[0222] 2) Add DC / HepG2(GT+)3×10 of Example 1 suspended in incomplete RIPM1640 medium to ea...
Embodiment 3
[0235] Example 3. Treatment of tumors by T lymphocytes secreting IFN-γ induced by the fusion cell DC / HepG2 (GT-Eng+)
[0236] The experiment was repeated three times, and the specific steps for each repeated experiment were as follows:
[0237] T lymphocytes secreting IFN-γ induced by DC / HepG2 (GT+) in Example 2, T lymphocytes secreting IFN-γ induced by DC / HepG2 (GT-Eng+), and IFN-γ secreted by DC / HepG2 T lymphocytes, T lymphocytes secreting IFN-γ induced by DC / HepG2 (Eng+), T lymphocytes secreting IFN-γ induced by DC / HepG2 (pLVX-Puro), T lymphocytes secreting IFN-γ induced by DC Lymphocytes, T lymphocytes induced by HepG2, T lymphocytes induced by HepG2 (Eng+), T lymphocytes induced by HepG2 (GT+) and T lymphocytes induced by HepG2 (pLVX-Puro) were suspended in PBS, and the cells were obtained separately The content is 10 5 A / μL DC / HepG2(GT+)-induced IFN-γ-secreting T lymphocyte suspension, DC / HepG2(GT-Eng+)-induced IFN-γ-secreting T lymphocyte suspension, DC / HepG2-induced secret...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com