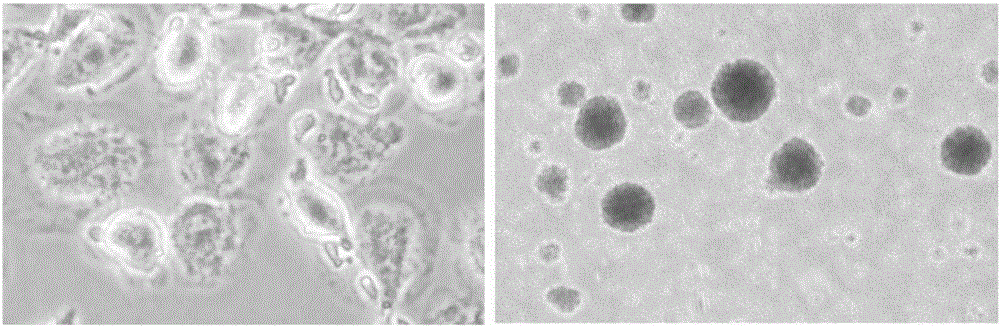
Method for cryopreservation of DC cells and CIK seed cells in blood, prepared cells and application
A seed cell and cryopreservation technology, which is applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., can solve the problems of patient injury and inappropriate collection of peripheral blood, so as to improve recovery efficiency and avoid the risk of FBS The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] Preparation of autologous serum:
[0024] Collect 80-120ml of peripheral blood or umbilical cord blood, add physiological saline at a volume ratio of 1:1, mix well, and separate plasma and mononuclear cells through Ficoll lymphocyte separation medium;
[0025] Inactivate the plasma at 55-58°C for 30 minutes, centrifuge the inactivated plasma at 4500g-6000g for 8-12 minutes, filter the supernatant with a 0.22um filter membrane and finally prepare autologous serum;
[0026] Cryopreservation of mononuclear cells:
[0027] 1. After the isolated mononuclear cells were washed once with normal saline containing 1% FBS, the cells were resuspended in immune cell culture medium and counted;
[0028] 2. Prepare DC cell induction medium: the immune cell medium contains FBS with a volume fraction of 5-8%, IL-4 with a final concentration of 50-100IU / ml, and GM-CSF with a final concentration of 500-1500IU / ml;
[0029] 3. Take 1-2*10 according to the cell counting result 7 Inoculate...
Embodiment 1
[0042] Preparation of autologous serum:
[0043]1. Collect 120ml of peripheral blood, add physiological saline at a volume ratio of 1:1, mix well, and separate plasma and mononuclear cells through Ficoll lymphocyte separation medium;
[0044] 2. Inactivate the plasma at 56°C for 30 minutes, centrifuge the inactivated plasma at 4500gg for 12 minutes, filter the supernatant with a 0.22um filter membrane and finally prepare 223ml of autologous serum;
[0045] Cryopreservation of mononuclear cells:
[0046] 1. After the isolated mononuclear cells were washed once with 30ml of normal saline containing 1% FBS, the cells were resuspended in 20ml of immune cell culture medium and counted to obtain 1.98*10 8 cells;
[0047] 2. Prepare DC cell induction medium: the immune cell culture medium contains FBS with a volume fraction of 5%, IL-4 with a final concentration of 100IU / ml, and GM-CSF with a final concentration of 1000IU / ml;
[0048] 3. Take 2*10 according to the cell counting re...
Embodiment 2
[0061] Preparation of autologous serum:
[0062] 1. Collect 80ml of peripheral blood or umbilical cord blood, add physiological saline at a volume ratio of 1:1, mix well, and separate plasma and mononuclear cells through Ficoll lymphocyte separation medium;
[0063] 2. Inactivate the plasma at 55°C for 30 minutes, centrifuge the inactivated plasma at 5000g for 8 minutes, filter the supernatant with a 0.22um filter membrane and finally prepare autologous serum;
[0064] Cryopreservation of mononuclear cells:
[0065] 1. After the isolated mononuclear cells were washed once with 20ml of normal saline containing 1% FBS, the cells were resuspended in immune cell medium and counted to obtain 1.5*10 8 cells;
[0066] 2. Prepare DC cell induction medium: the immune cell culture medium contains FBS with a volume fraction of 6%, IL-4 with a final concentration of 50IU / ml, and GM-CSF with a final concentration of 500IU / ml;
[0067] 3. Take 1*10 according to the cell counting result ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com