CETP antigenic peptide and fusion protein and their composition and applications
A fusion protein and antigenic peptide technology, applied in the field of antigenic peptides, can solve the problems of immunogenicity and insufficient efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0109] Materials and Method
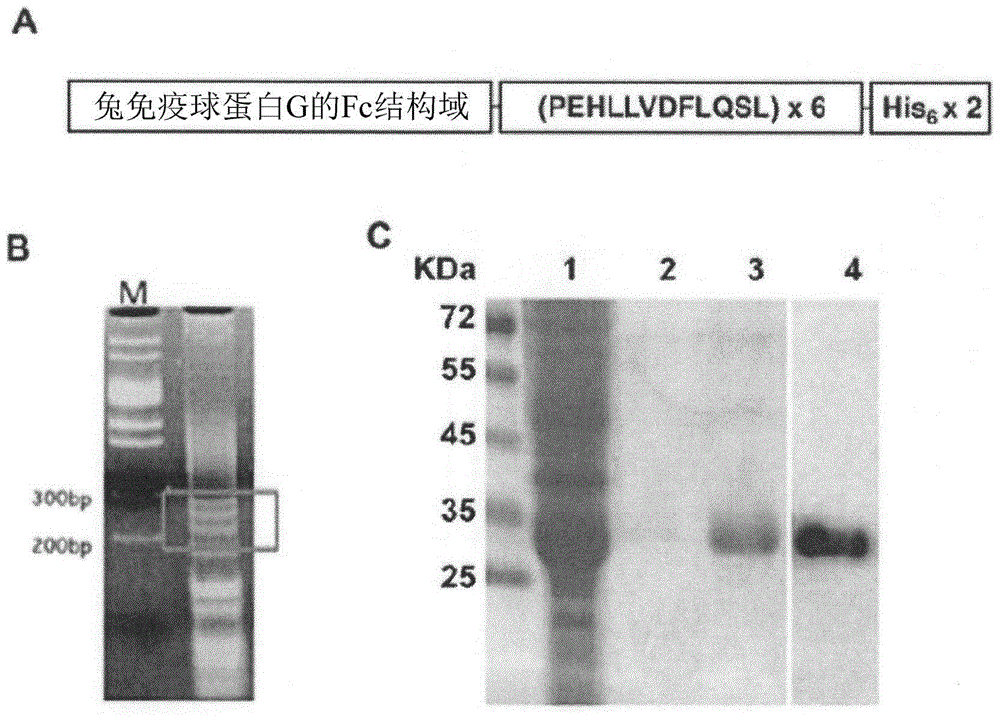
[0110] A DNA fragment of 6 repeated sequences encoding the human CETP epitope was constructed, and then it was fused with rabbit Fc and the fusion protein was expressed in E. coli BL21 (DE3).
[0111] As previously described (Hsu et al. 2000, Cancer Res. 60:3701-3705), the peptide PEHLLVDFLQSL encoding the human CETP epitope was generated by template repeat polymerase chain reaction (TR-PCR) (Gaofu et al. 2004, Vaccine 22: 3187-3194) DNA fragments of 6 repeating sequences ( figure 1 ). The adapter primers (Table 1) were then used to subject the TR-PCR product to adapter PCR to form restriction sites at the 5'end and 3'end to facilitate further subcloning. The 200-300bp linker PCR product ( figure 1 B) Eluted and cloned into T-Easy vector (Promega). The clones containing 6 copies of the CETP epitope were identified by sequencing and subcloning at the 3'end of the region encoding the Fc domain of rabbit IgG into a modified plasmid pET21b vector (Novage...
example 1
[0135] Example 1 Production of Fc-CETP6 vaccine
[0136] Generate DNA encoding 6 repeats of CETP epitope and induce and express the fusion protein in E. coli strain BL21 (DE3), such as figure 1 Shown in. The fusion protein Fc-CETP6 was purified by affinity chromatography on a histidine (His) binding column. The purity of the Fc-CETP6 fusion protein was checked by Coomassie Blue staining and Western blotting, and the purity reached 95%. The Fc-CETP purified by histidine binding 6 Purity: Lanes 1 to 3 show the staining results of Coomassie Blue for flow-through, washing and elutes, respectively, while Lane 4 shows that the eluate uses anti-rabbit IgG (Fc) Western blot analysis ( figure 1 C).
example 2
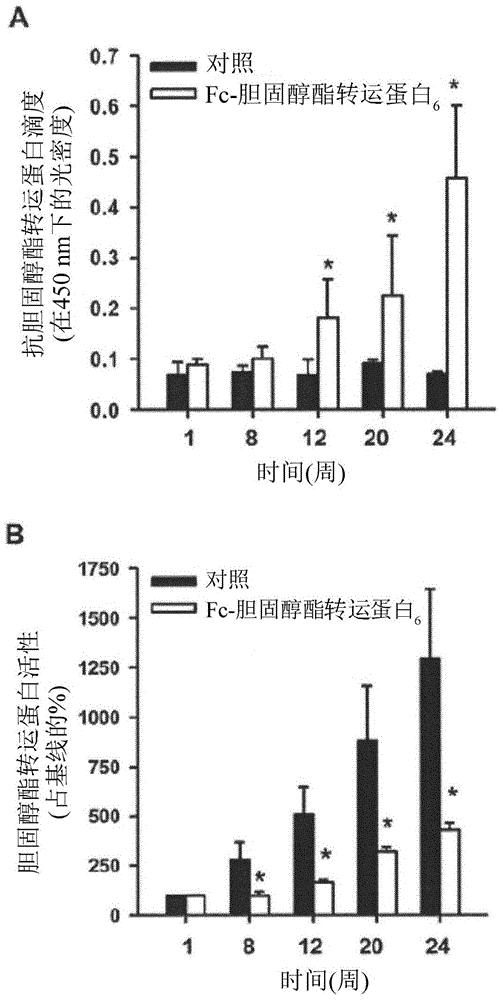
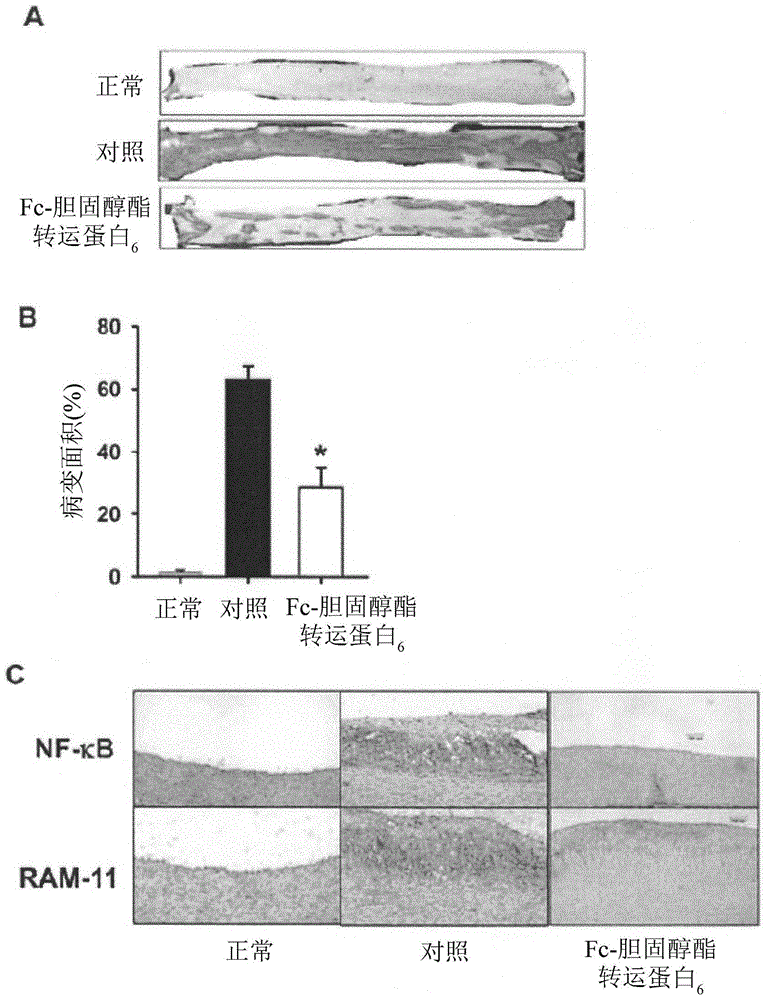
[0137] Example 2. In rabbits fed with HFC feed, Fc-CETP 6 The vaccine raises antibodies against CETP and reduces CETP activity
[0138] In order to confirm that the anti-CETP antibody is produced and the antibody reacts with CETP in the circulation, plasma anti-CETP titer and plasma CETP activity are measured. figure 2 A shows that in Fc-CETP 6 Anti-CETP antibodies were detected in the group from week 12 and the titer increased until the end of the study at week 24. figure 2 B shows that in the two groups fed with HFC feed, plasma CETP activity increased in a time-dependent manner, but in Fc-CETP 6 In the group, the plasma CETP activity was significantly lower. These results show that the injection of Fc-CETP 6 The production of antibodies against CETP is induced, which then reduces CETP activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com