Material for organic electroluminescent elements, and organic electroluminescent element using same
一种发光元件、有机电场的技术,应用在发光材料、电气元件、有机化学等方向,能够解决未示出碳硼烷化合物有用性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
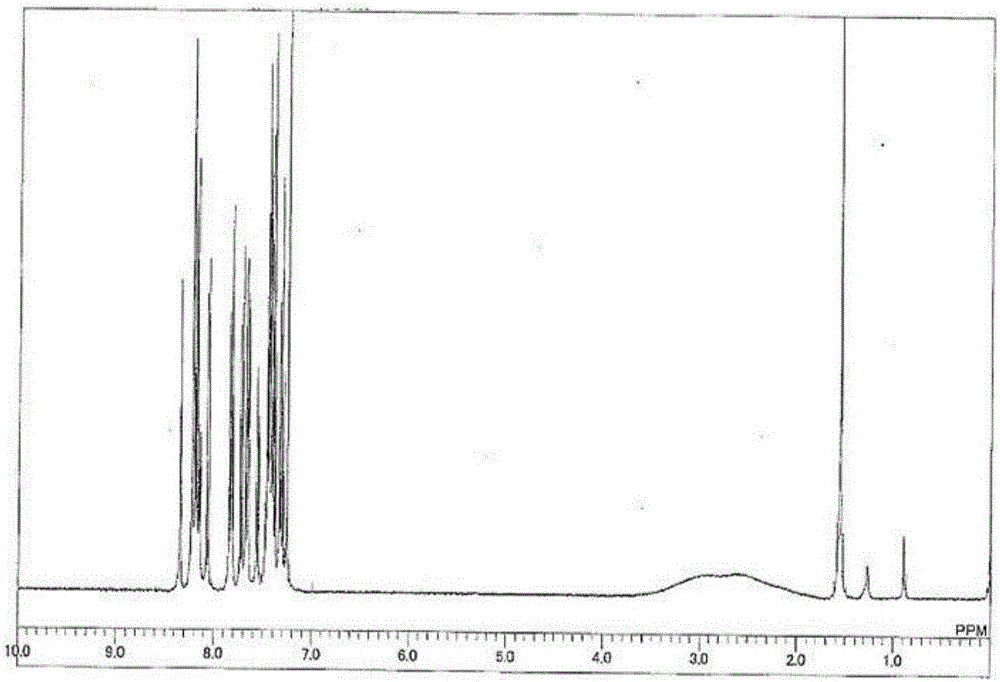
[0133] Compound 1 was synthesized according to the following reaction formula.
[0134] [chemical 13]
[0135]
[0136] Under a nitrogen atmosphere, 35.0 g (0.243 mol) of m-carborane and 200 mL of 1,2-dimethoxyethane (DME) were added, and the resulting DME solution was cooled to 0°C. 96.8 mL of a 2.69 M n-butyllithium hexane solution was added dropwise, followed by stirring for 30 minutes while cooling in an ice bath. After adding 70 mL of pyridine and stirring at room temperature for 10 minutes, 75.6 g (0.763 mol) of copper (I) chloride was added and stirred at 65° C. for 30 minutes. Then, 73.9 g (0.280 mol) of 2-bromodibenzothiophene was added, and it stirred at 95 degreeC overnight. After cooling the reaction solution to room temperature, the precipitated crystals were collected by filtration, and the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain 8.0 g (24.5 mmol, yield 10.0%) of Inte...
Embodiment 2
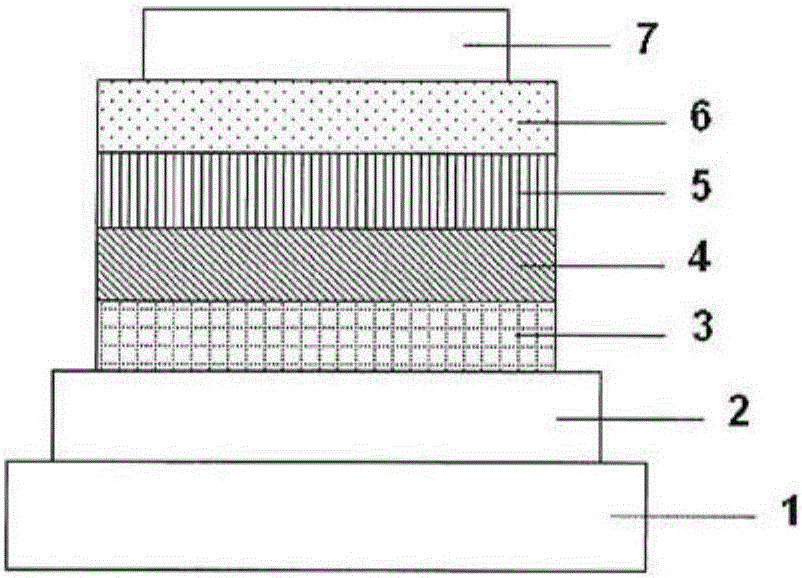
[0144] On a glass substrate formed with an anode of indium tin oxide (ITO) with a film thickness of 70 nm, the -5Pa vacuum degree to laminate each film. First, copper phthalocyanine (CuPC) was formed as a hole injection layer on ITO with a thickness of 30 nm. Next, diphenylnaphthyldiamine (NPD) was formed to a thickness of 15 nm as a hole transport layer. Then, on the hole transport layer, compound 1 as the host material of the light-emitting layer and iridium complex [bis(4,6 -di-fluorophenyl)-pyridinate-N, C2']picolinate (III)] (Iridium(III)bis(4,6-di-fluorophenyl)-pyridinate-N,C2']picolinate, FIrpic), The light emitting layer was formed with a thickness of 30 nm. The concentration of FIrpic was 20%. Then, Alq was formed with a thickness of 25nm 3 as an electron transport layer. Furthermore, lithium fluoride (LiF) was formed as an electron injection layer with a thickness of 1.0 nm on the electron transport layer. Finally, aluminum (Al) was formed as an electrode with...
Embodiment 3~ Embodiment 9
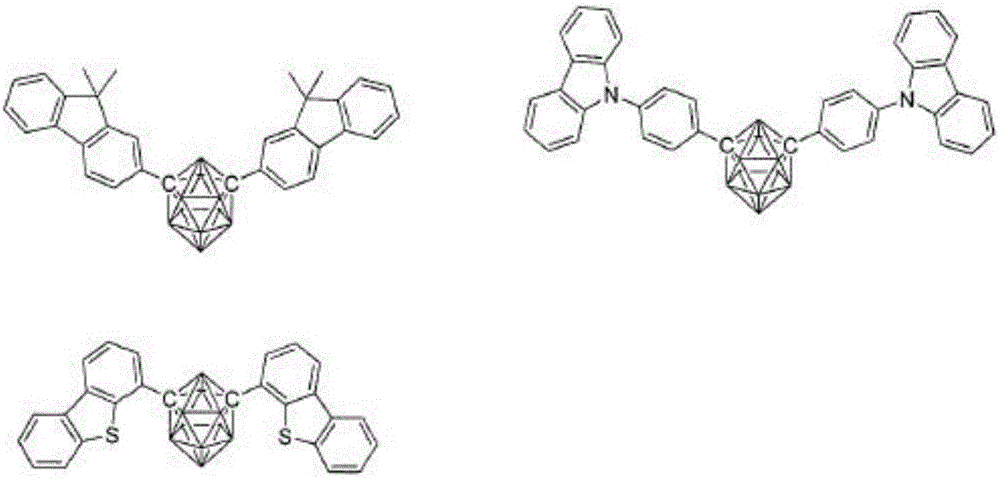
[0147] Compound 13, Compound 25, Compound 26, Compound 29, Compound 36, Compound 45, and Compound 57 were used instead of Compound 1 as the host material of the light-emitting layer in Example 2, and an organic EL was produced in the same manner as in Example 2. element.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com