Chain olefin-cycloolefin copolymer
A cyclic olefin copolymer and alkene technology, which is applied in the olefin-cycloolefin copolymer and its application field, can solve the problems of tensile stress, insufficient elongation at break, etc., and achieve excellent physical and mechanical properties, good processing performance, low ash effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0272] The following examples will be used to illustrate the present invention in further detail, but the present invention is not limited to these examples.
[0273] The measurement or evaluation of each physical property in an Example and a comparative example was performed as follows.
[0274]1. The content of structural unit B existing in the form of diads
[0275] The content of structural unit B in the copolymer is passed 13 C-NMR measurement.
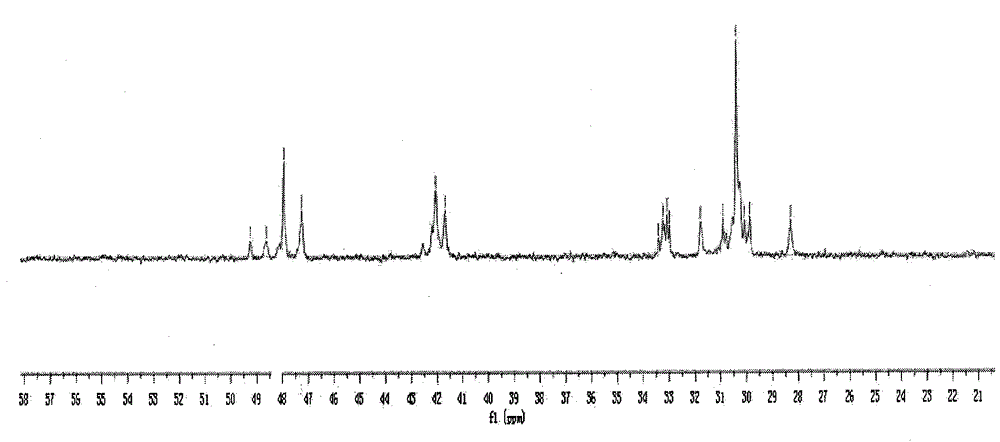
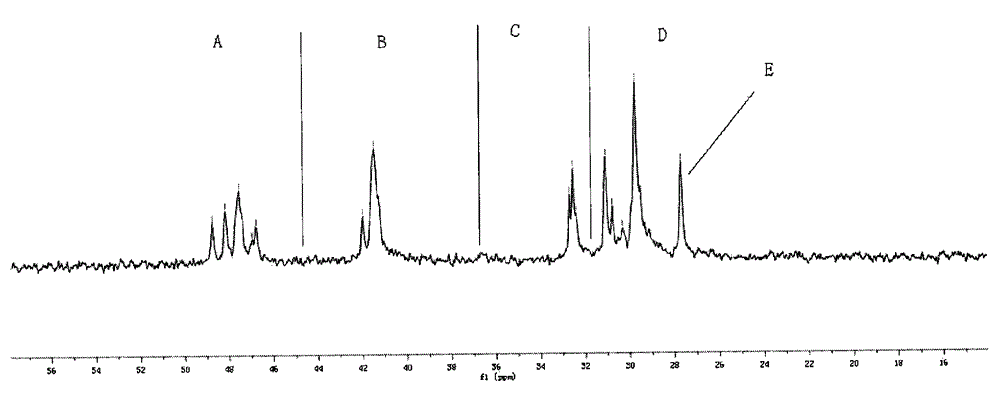
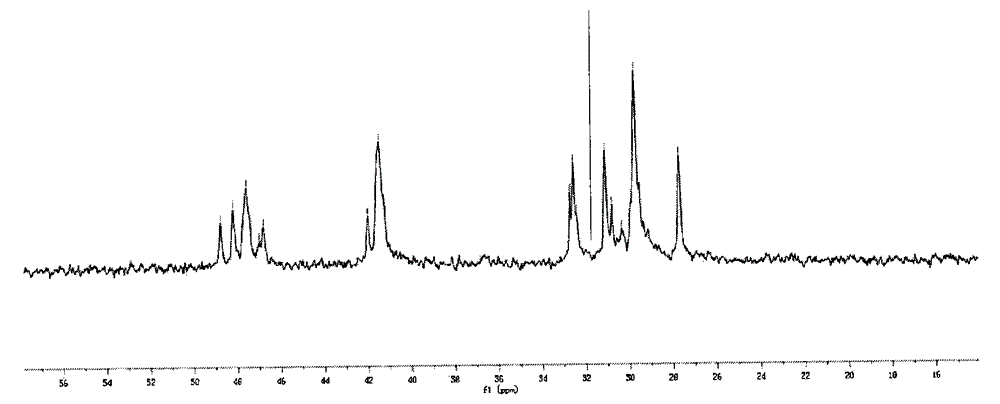
[0276] In the following examples, ethylene was used as the alkene and norbornene was used as the cyclic olefin. For the resulting ethylene-norbornene copolymer, refer to figure 1 , the content of the structural unit B existing in the form of diads is calculated as follows.
[0277]
[0278] Among them, X N(全部) Indicates the content of all structural units derived from norbornene in the copolymer, X N(二连续NN中的N) Indicates the content of structural units derived from norbornene in the form of dyads, X (单独N) Indicates the c...
manufacture Embodiment 1
[0296] In a dry 500ml three-necked flask, add 2-aminoanisole sulfide (0.2mol), absolute ethanol (160ml), 3,5-di-tert-butyl salicylaldehyde (0.2mol), acetic acid (0.3ml) successively , heated up to reflux temperature and reacted for 2hr, cooled to room temperature, filtered, washed three times with absolute ethanol, and dried in vacuum to obtain 49.7g of 2-aminoanisole sulfide 3,5-di-tert-butyl salicylaldehyde, called Ligand L1. Elemental analysis: C 74.57% (theoretical value 74.32%); H 8.35% (theoretical value 8.22%); N 4.07% (theoretical value 3.94%). 1 H NMR δ=13.4(OH), 8.6(CHN), 7.5-7.1(Ar-H), 3.25(SCH 3 ), 1.45 (C (CH 3 ) 3 ), 1.35 (C (CH 3 ) 3 )
[0297]
manufacture Embodiment 2
[0299] In a dry 500ml three-necked flask, add 2-aminoanisole sulfide (0.2mol), absolute ethanol (160ml), 3,5-di-tert-butyl salicylaldehyde (0.2mol), acetic acid (0.3ml) successively , heated to reflux temperature and reacted for 2 hours, cooled to room temperature, filtered, washed three times with absolute ethanol, and dried in vacuum to obtain 49.7 g of 2-aminoanisole sulfide 3,5-di-tert-butyl salicylaldehyde.
[0300] Slowly add a diethyl ether solution of lithium aluminum hydride (0.2 mol) to the toluene solution of 2-aminoanisole sulfide 3,5-di-tert-butyl salicylaldehyde (0.1 mol), and continue stirring for 30 minutes after the addition is complete. The reaction was terminated by adding ice water to the reaction solution. Purified by column chromatography to obtain a compound represented by the following formula, which is called ligand L2. Elemental analysis: C 73.67% (theoretical value 73.90%); H 8.71% (theoretical value 8.74%); N 4.08% (theoretical value 3.92%). 1 H N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com