Method for synthesizing prothioconazole and optical active body thereof and intermediate
An optically active, prothioconazole technology, which is applied in the field of organic synthesis and achieves the effects of high yield, few by-products and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
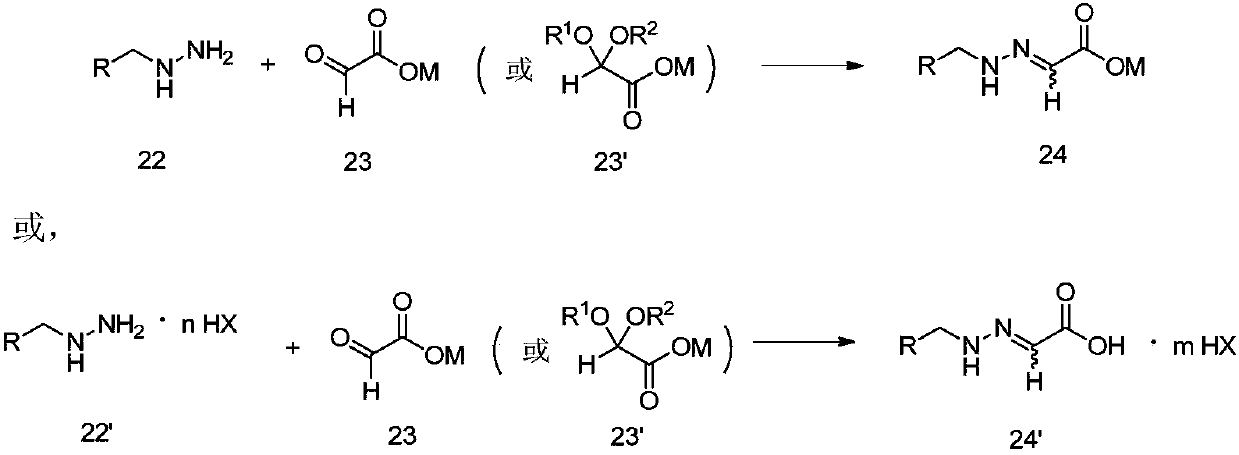
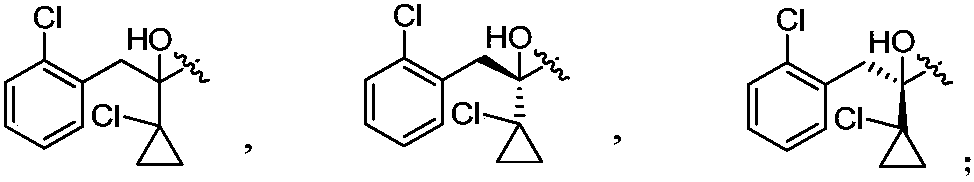
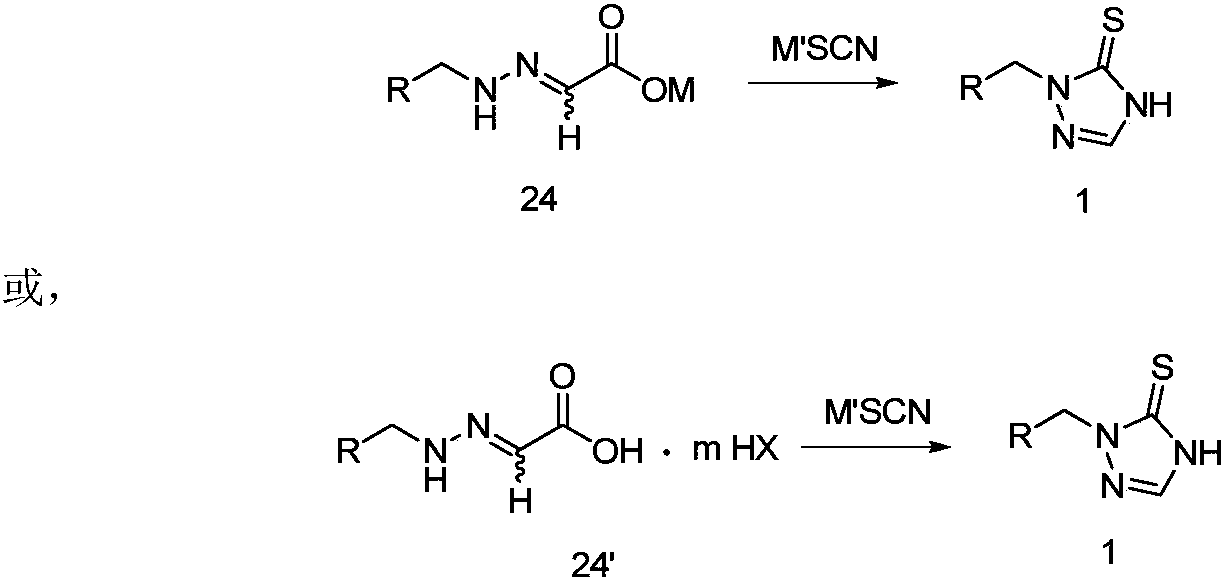
[0073] Example 1: Synthesis of 2-{2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]hydrazono}acetic acid
[0074] Add 15.5g of 2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-hydrazinopropan-2-ol hydrochloride, 120mL water and 15mL acetonitrile in a 250mL reaction flask, drop Add 7.5g of 50% glyoxylic acid aqueous solution, stir at room temperature, filter after the reaction, wash with water, and dry to obtain 16.4g of solid product (99% yield). 1 H NMR (δ, CDCl 3 ):7.456-7.437(m,1H),7.405-7.386(m,1H),7.271-7.252(m,1H),7.245-7.223(m,1H),6.971-6.899(t,1H),6.781(s ,1H),3.906-3.898,3.878-3.869(dd,1H),3.547-3.519(d,1H),3.519-3.508,3.491-3.480(dd,1H),3.211-3.183(d,1H),2.506( s,2H),1.175-1.134(m,1H),0.964-0.852(m,3H); MS: m / z=330.9([M+1] + ).
Embodiment 2
[0075] Example 2: Synthesis of (2R)-2-{2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]hydrazono}acetic acid
[0076] Add 13.7g (2R)-2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-hydrazinopropan-2-ol, 100mL water and 20mL acetonitrile in a 250mL reaction flask, drop Add 7.5g of 50% glyoxylic acid aqueous solution, stir at room temperature, filter after the reaction, wash with water, and dry to obtain 15.2g of solid product (yield: 92%).
Embodiment 3
[0077] Example 3: Synthesis of (2S)-2-{2-[2-(1-chlorocyclopropyl)-3-(2-chlorophenyl)-2-hydroxypropyl]hydrazono}acetic acid
[0078] Add 13.7g (2S)-2-(1-chlorocyclopropyl)-1-(2-chlorophenyl)-3-hydrazinopropan-2-ol, 100mL water and 20mL acetonitrile in a 250mL reaction flask, drop Add 7.5 g of 50% glyoxylic acid aqueous solution, stir at room temperature, filter after the reaction, wash with water, and dry to obtain 15.0 g of solid product (yield 91%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com