Benazepril hydrochloride soft chewable tablet and preparation method thereof
A technology of benazepril hydrochloride and soft chewable tablets, which is applied in the fields of pharmaceutical formula, pill delivery, cardiovascular system diseases, etc. It can solve the problems of the stability of the main drug components, disintegration, and increased difficulty in dissolution, so as to improve the palatability performance, improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
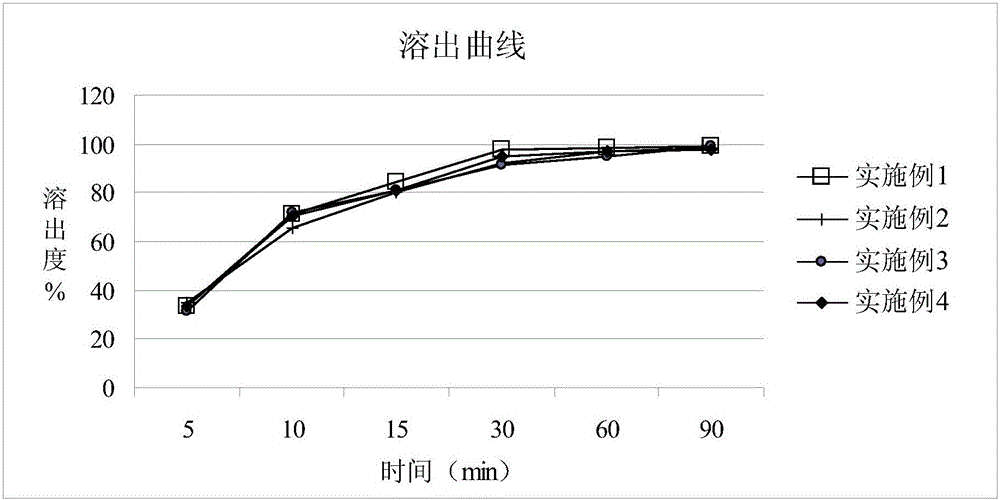
Image
Examples
Embodiment 1
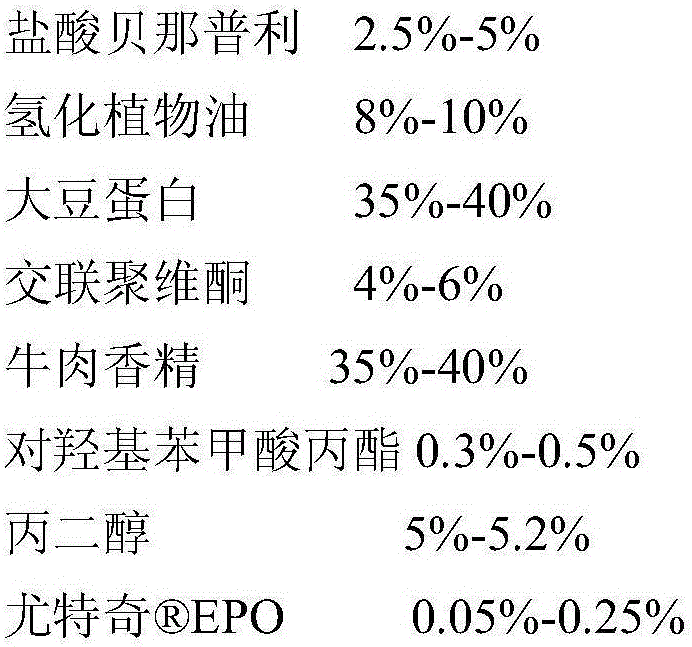
[0051] A soft chewable tablet of benazepril hydrochloride, calculated by weight percentage, the content of each component is as follows:
[0052]
[0053] The preparation method of benazepril hydrochloride soft chewable tablet: make benazepril hydrochloride utilize EPO is fluidized bed coated, the coating weight is increased by 5%, sieved through 40 mesh and 65 mesh, and the coated granules are obtained by removing coarse particles and uncoated fine powder; the coated granules are mixed with hydrogenated vegetable oil and soybean protein , cross-linked povidone, and artificial beef flavor are pre-mixed; then propyl p-hydroxybenzoate and propylene glycol are added for total mixing, mixed evenly and then extruded.
Embodiment 2
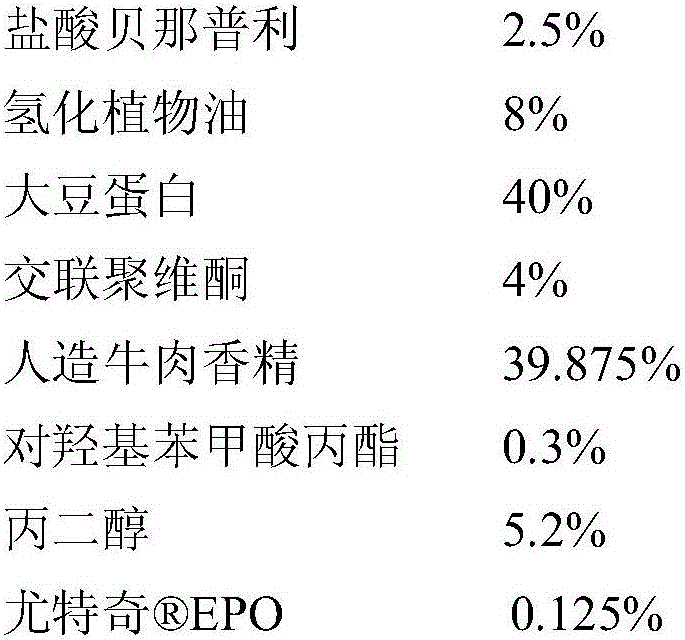
[0055] A soft chewable tablet of benazepril hydrochloride, calculated by weight percentage, the content of each component is as follows:
[0056]
[0057]
[0058] Preparation method: use benazepril hydrochloride EPO is used for fluidized bed coating, and the weight gain of the coating is 4%, and the granules are sieved through 40 mesh and 65 mesh, and the coarse particles and uncoated fine powder are removed to obtain the coated granules; the coated granules are mixed with hydrogenated vegetable oil and soybean protein Cross-linked povidone and artificial beef flavor are premixed; then propyl p-hydroxybenzoate and propylene glycol are added for total mixing, mixed evenly and then extruded.
Embodiment 3
[0060] A soft chewable tablet of benazepril hydrochloride, calculated by weight percentage, the content of each component is as follows:
[0061]
[0062] Preparation method: use benazepril hydrochloride EPO is fluidized bed coated, and the weight of the coating is increased by 5%; the granules are sieved through 40 mesh and 65 mesh, and the coarse particles and uncoated fine powder are removed to obtain the coated granules; the coated granules are mixed with hydrogenated vegetable oil and soybean protein Cross-linked povidone and artificial beef flavor are premixed; then propyl p-hydroxybenzoate and propylene glycol are added for total mixing, mixed evenly and then extruded.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com