Application of heat shock protein gp96 to treatment of systemic lupus erythematosus
A heat shock protein and lupus erythematosus technology, applied in the field of biomedicine, can solve the problems that SLE cannot be cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
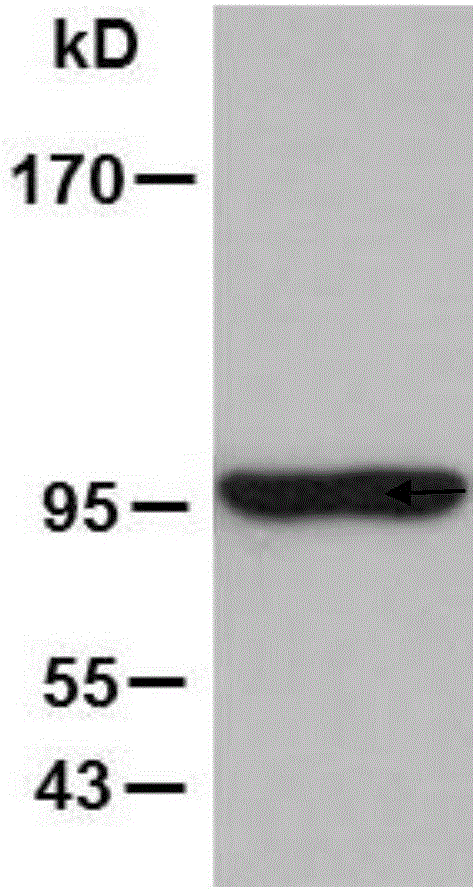
[0096] Example 1, the extraction of pgp96
[0097] The extraction steps of heat shock protein gp96 (hereinafter referred to as pgp96) in tissues are as follows:
[0098] (1) Isolate the placental tissue of the mouse before delivery to obtain the isolated mouse placental tissue. Take isolated mouse placental tissue or human isolated placental tissue, cut it into pieces, add solution A according to the mass volume ratio of 1g: 4mL, and then grind it with a glass homogenizer.
[0099] (2) After completing step (1), centrifuge at 16500 g for 1 h to obtain supernatant A.
[0100] (3) After completing step (2), take the supernatant A and centrifuge at 16500 g for 50 min to obtain the supernatant B.
[0101] (4) After completing step (3), take the supernatant B, add solution B at a volume ratio of 9:1, and mix well to obtain the sample solution.
[0102] (5) After completing step (4), load the sample solution onto the ConA Sepharose column.
[0103] (6) After step (5) is completed,...
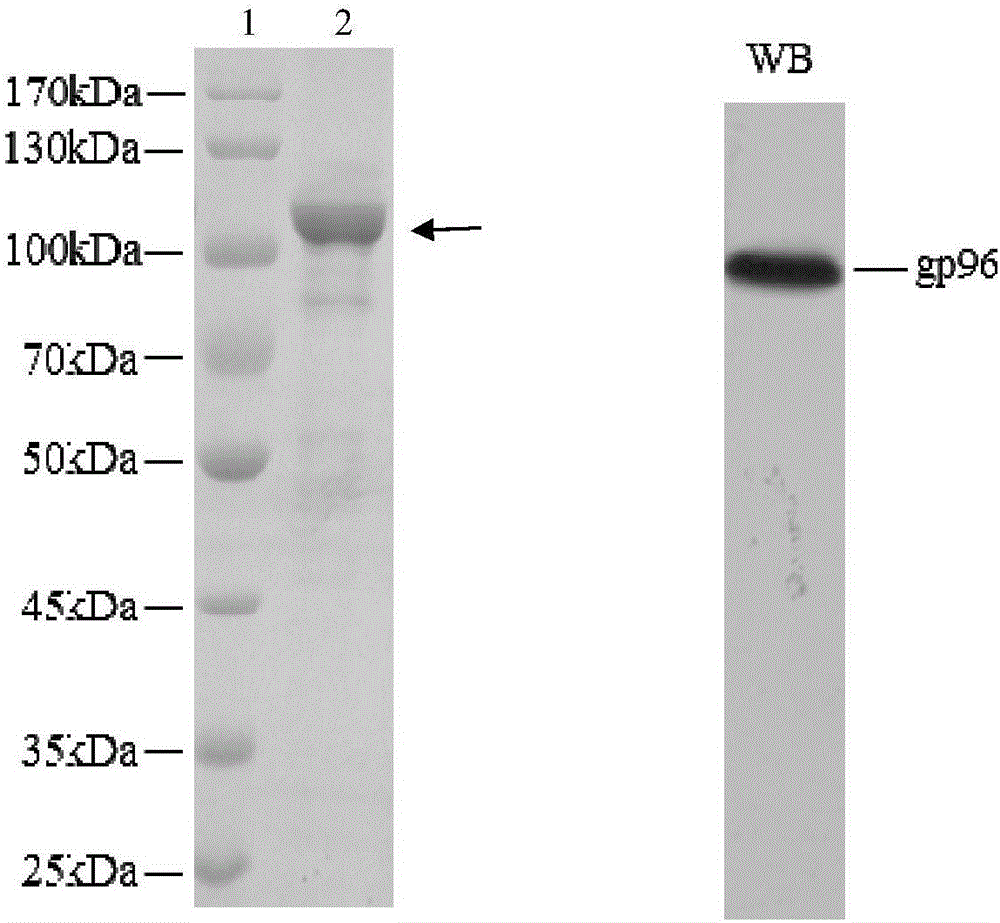
Embodiment 2
[0109] Embodiment 2, the preparation of recombinant heat shock protein gp96
[0110] 1. Recombinant plasmid pFastBac TM 1- Construction of gp96
[0111] 1. The RNA of HepG2 cells was extracted by Trizol method, and then cDNA was obtained by reverse transcription.
[0112] 2. According to the sequence of human gp96 gene (GenBank No. AY040226.1), artificially synthesized primer F1: 5'-G GAATTC ATGGACGATGAAGTTGAT-3' (the underline is the restriction endonuclease EcoRI recognition sequence) and R1: 5'-GC TCTAGA CTATTAGAATTCATCTTTTTC-3' (the underline is the recognition sequence of restriction endonuclease XbaI).
[0113] 3. After completing steps 1 and 2, use the cDNA obtained in step 1 as a template and F1 and R1 synthesized in step 2 as primers to perform PCR amplification to obtain PCR amplification products.
[0114] 4. Double-digest the PCR amplification product with restriction endonucleases EcoRI and XbaI, and recover the digested product.
[0115] 5. Digest plasmid ...
Embodiment 3
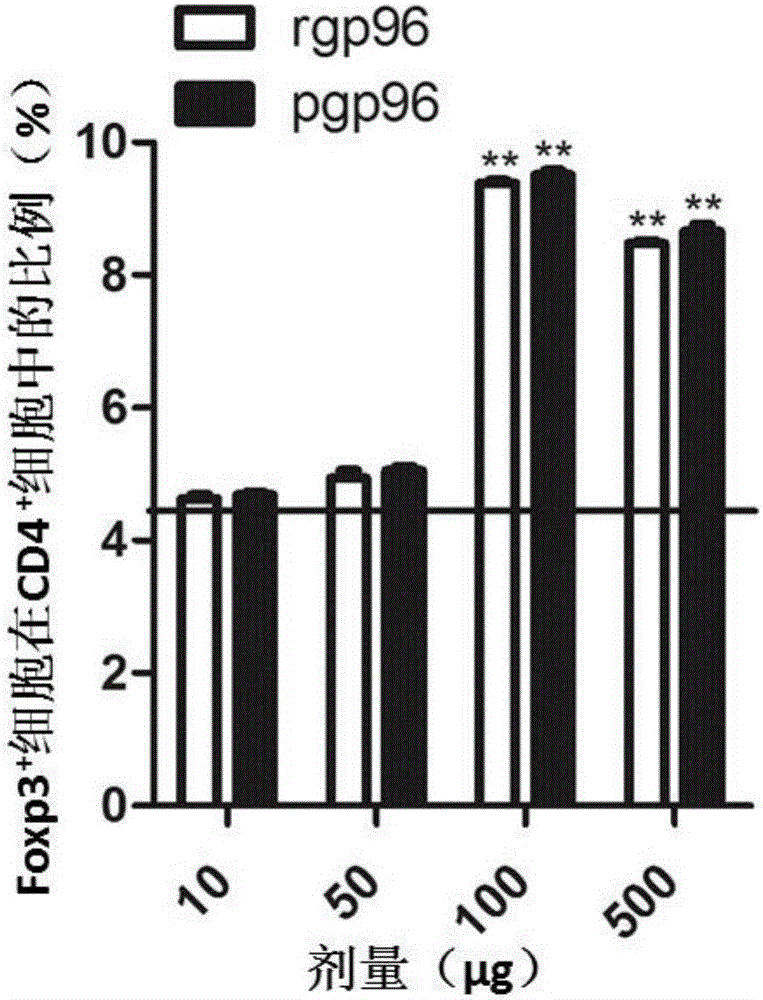
[0132] Example 3, Determination of the dosage of pgp96 or rgp96 in activated regulatory T cells
[0133] 1. Immunization of mice in groups
[0134] Ninety nine-week-old mice with a body weight of 18-22 g were randomly divided into pgp96 treatment group 1, pgp96 treatment group 2, pgp96 treatment group 3, pgp96 treatment group 4, rgp96 treatment group 1, rgp96 treatment group 2, rgp96 treatment group 3 , rgp96 treatment group 4 and control group (every group of 10), carry out the following treatments respectively:
[0135] pgp96 treatment group 1: on the first day of the experiment, the solution of pgp96 prepared in Example 1 was injected intraperitoneally; on the eighth day of the experiment, the solution of pgp96 prepared in Example 1 was injected intraperitoneally again; on the 22nd day of the experiment, the solution prepared in Example 1 was injected again intraperitoneally A solution of pgp96. The dose of each injection was 10 μg pgp96 per mouse.
[0136] pgp96 treatme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com