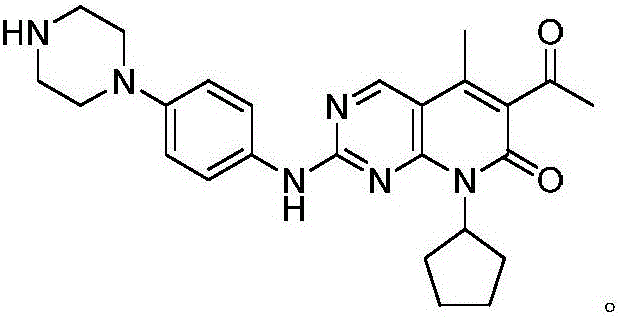
Method for preparing palbociclib intermediate
An intermediate, cyclopentyl technology, applied in the field of pharmaceutical synthesis, can solve the problems of long reaction time, low overall yield, unfavorable health of workers, etc., and achieves the effects of mild reaction conditions and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
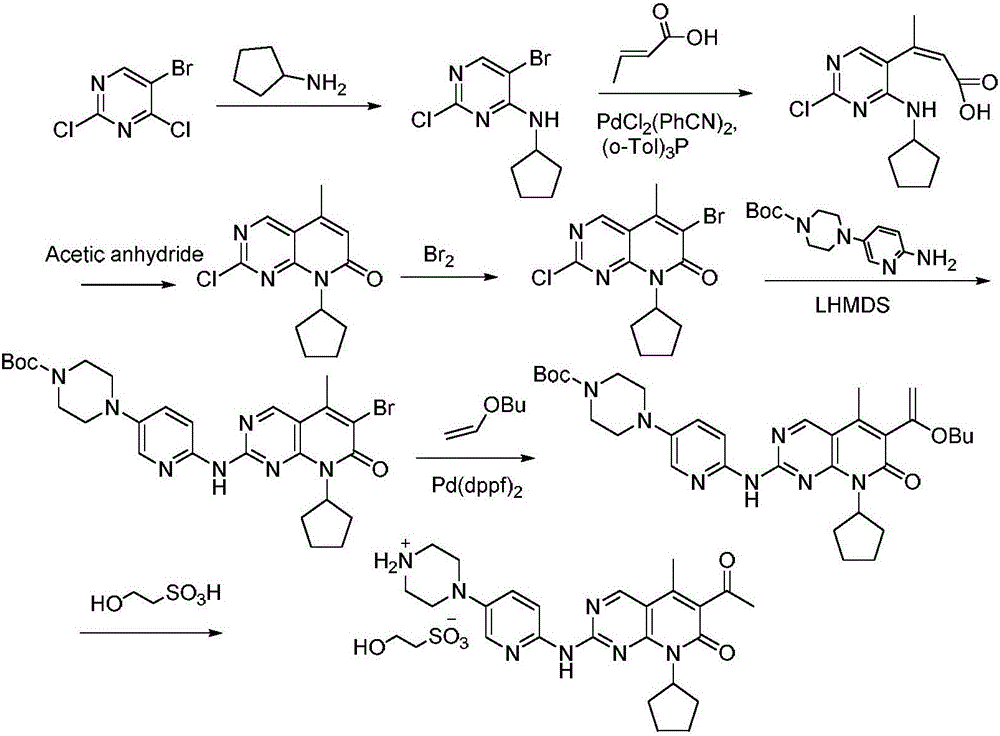
[0031] A method for preparing palbociclib intermediates, the method comprising the following steps:
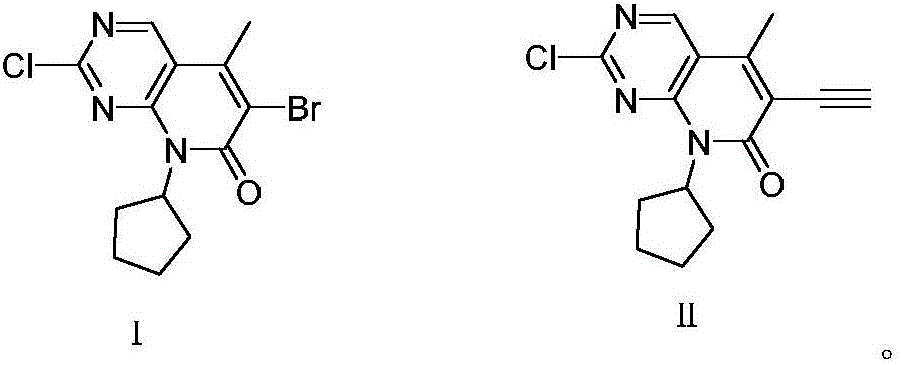
[0032] 1) Under the protection of nitrogen, the compound N-cyclopentyl-5-methyl-2-chloro-6-bromopyrido[2,3-d]pyrimidin-7(8H)-one represented by formula (I) 34.3g (100mmol) reacted with 19.6g (200mmol) of trimethylsilyl acetylene in THF at 45-50°C for 3 hours in the presence of 5.7g (40mmol) of cuprous bromide and 16.8g (150mmol) of potassium tert-butoxide, After the reaction, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain the compound N-cyclopentyl-5-methyl-2-chloro-6-ethynylpyrido[2, 3-d] Pyrimidin-7(8H)-one 23.7g, yield 82.4%, purity 99.96% (HPLC area normalization method).
[0033] 2) The compound N-cyclopentyl-5-methyl-2-chloro-6-ethynylpyrido[2,3-d]pyrimidine-7(8H) obtained in step 1) represented by formula (II) Add 10 g of ketone into acidic aqueous solution (10% sulfuric acid aqueous solution) an...
Embodiment 2
[0036] A method for preparing palbociclib intermediates, the method comprising the following steps:
[0037] 1) Under the protection of nitrogen, the compound N-cyclopentyl-5-methyl-2-chloro-6-bromopyrido[2,3-d]pyrimidin-7(8H)-one represented by formula (I) In the presence of 34.3g (100mmol) of cuprous bromide 5g (35mmol) and potassium tert-butoxide 17.9g (160mmol), react with 14.7g (150mmol) of trimethylsilylacetylene in THF at 65°C for 4 hours, after the reaction ends , evaporate the solvent under reduced pressure, wash with water, recrystallize from methanol, and dry to obtain the compound N-cyclopentyl-5-methyl-2-chloro-6-ethynylpyrido[2,3-d ] Pyrimidin-7(8H)-one 23.4g, yield 81.3%, purity 99.95% (HPLC area normalization method).
[0038] 2) The compound N-cyclopentyl-5-methyl-2-chloro-6-ethynylpyrido[2,3-d]pyrimidine-7(8H) obtained in step 1) represented by formula (II) - 10g of ketone was added to acidic aqueous solution (15% sulfuric acid aqueous solution) and hydroly...
Embodiment 3
[0040] A method for preparing palbociclib intermediates, the method comprising the following steps:
[0041] 1) Under the protection of nitrogen, the compound N-cyclopentyl-5-methyl-2-chloro-6-bromopyrido[2,3-d]pyrimidin-7(8H)-one represented by formula (I) In the presence of 7.2g (50mmol) of cuprous bromide (50mmol) and 17.9g (160mmol) of potassium tert-butoxide, 34.3g (100mmol) reacted with 19.6g (200mmol) of trimethylsilyl acetylene in THF at 60°C for 3 hours, and the reaction ended Afterwards, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain the compound N-cyclopentyl-5-methyl-2-chloro-6-ethynylpyrido[2,3- d] Pyrimidin-7(8H)-one 22.9g, yield 79.6%, purity 99.91% (HPLC area normalization method).
[0042] 2) The compound N-cyclopentyl-5-methyl-2-chloro-6-ethynylpyrido[2,3-d]pyrimidine-7(8H) obtained in step 1) represented by formula (II) - 10g of ketone was added to acidic aqueous solution (10% sulfuric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com