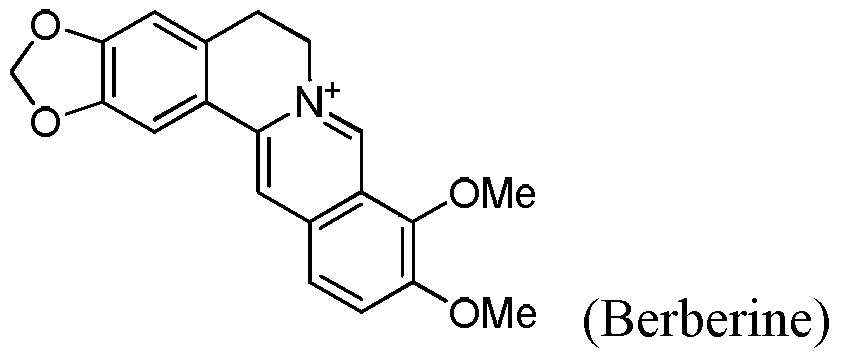
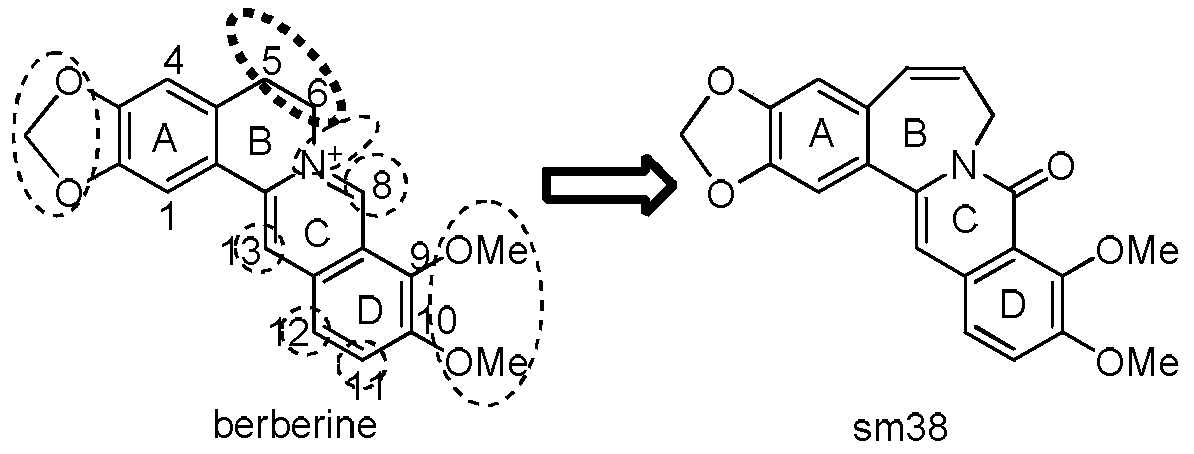
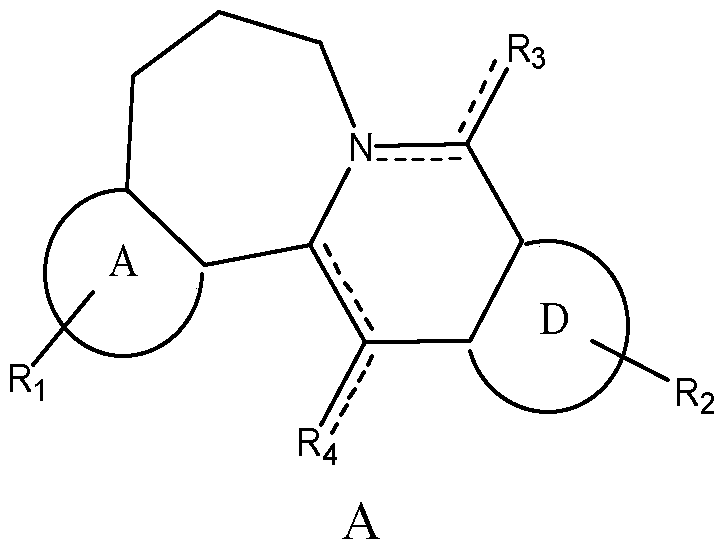
Seven-membered ring berberine analogue and its pharmaceutical composition, preparation method and application
A technology of berberine and analogs, which is applied in the fields of medicinal chemistry and pharmacotherapy, can solve the problems of large dosage, low oral bioavailability of berberine, affecting bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Preparation of compound 2
[0127]
[0128] Method 1: Compound 1 (refer to the method of Chinese Patent Application No. 201010537594.1 for specific preparation) is dissolved in methanol, a catalytic amount of 10% palladium carbon is added, hydrogen gas is introduced, and the reaction is carried out at room temperature until the raw materials are completely reacted. The reaction solution was filtered with celite, and the filtrate was evaporated to dryness under reduced pressure to obtain 2 as a white solid with a yield of 95%.
[0129] Method 2: Compound 1 (1.0eq.) was dissolved in methanol, a catalytic amount of 10% palladium on carbon was added, and ammonium formate (10 equivalents) was added, and the reaction was heated under reflux until the reaction of the raw materials was complete. The reaction solution was filtered with celite, the filtrate was evaporated to dryness under reduced pressure, and separated on a silica gel column (PE / EA=2:1) to obtain 2 as a whi...
Embodiment 2
[0132] Preparation of Compounds 3a-3d
[0133]
[0134] Preparation of 3a: Compound 2 (1.0eq.) was dissolved in carbon tetrachloride, under nitrogen protection, added NBS (nitrobromosuccinimide, 1.2eq.), heated to 50°C for 5 hours, filtered, tetrachloro After washing with carbon dioxide, the filtrate was evaporated to dryness under reduced pressure, and separated on a silica gel column (PE / EA=2:1) to obtain a white solid 3a with a yield of 80%.
[0135] 1 H NMR (400MHz, CDCl 3 )δ7.94–7.82(d,J=9.1Hz,1H),7.47–7.38(d,J=9.0Hz,1H),7.11(s,1H),6.76(s,1H),6.08(s,1H ),6.05(s,1H),5.17–5.03(m,1H),4.03(s,3H),4.00(s,3H),3.05–2.94(m,1H),2.67–2.49(m,2H), 2.39–2.27(m,1H),1.84–1.72(m,1H). 13 CNMR (126MHz, CDCl 3 )δ159.09,152.24,149.23,148.72,145.52,139.44,133.28,130.74,127.08,123.21,120.06,118.32,111.96,108.53,101.47,99.65,77.24,61.60,56.65,42.41,29.47,28.66.MS(ESI)m / z[M+H] + 444.1.
[0136] Preparation of 3b: Compound 2 (1.0eq.) was dissolved in carbon tetrachloride, under nitro...
Embodiment 3
[0143] Preparation of Compound 4
[0144]
[0145] Compound 3a (1.0eq.) was dissolved in dichloromethane, added 10% solution volume of concentrated hydrochloric acid, stirred and reacted for 2 hours, the reaction solution was diluted with water, extracted with dichloromethane, separated organic phase, washed with water, washed with saturated sodium chloride, no Dry over sodium sulfate and evaporate to dryness under reduced pressure to obtain white solid 4 with a yield of 93%.
[0146] 1 H NMR (400MHz, CDCl 3 )δ13.48(s,1H),7.52–7.41(m,1H),7.41–7.32(m,1H),7.12(s,1H),6.75(s,1H),6.12(s,1H),6.05 (s,1H),5.11–4.94(m,1H),4.01(s,3H),3.12–2.96(m,1H),2.73–2.58(m,1H),2.59–2.47(m,1H),2.36 –2.18(m,1H),1.88–1.74(m,1H). 13 C NMR (126MHz, CDCl 3 )δ164.60,150.46,148.95,145.99,145.69,138.14,133.14,129.22,126.35,118.31,116.41,112.09,110.97,108.57,102.76,101.57,77.24,56.53,41.90,29.34,28.55.MS(ESI)m / z [M+H] + 430.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com