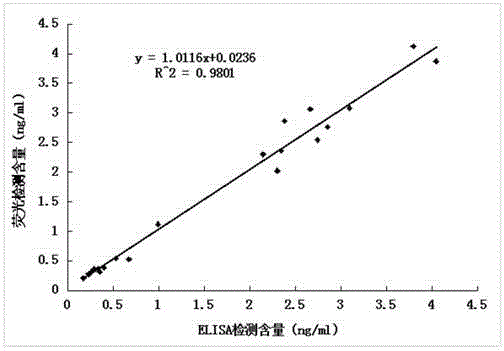
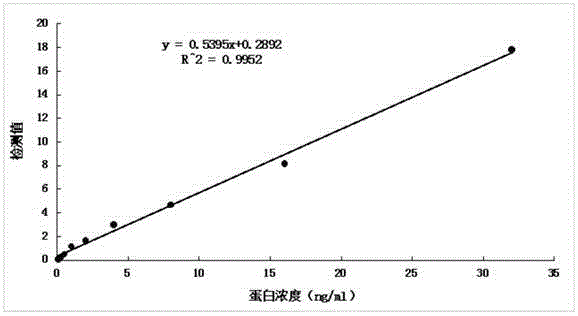
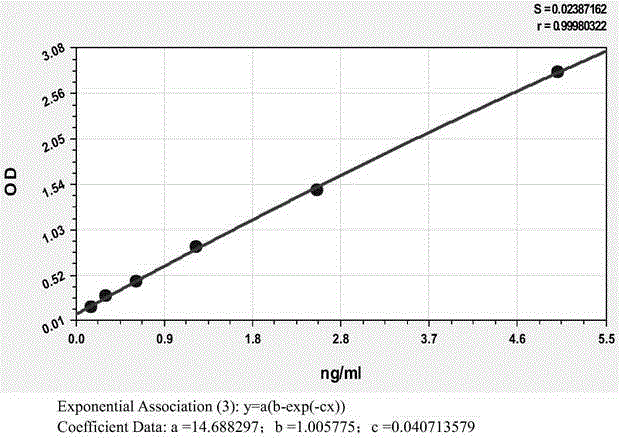
Anti-human kininogenase antibody and application thereof
A kininogenase and antibody technology, applied in the field of immunochemistry, can solve the problem that specific antibodies cannot effectively recognize natural hK1, etc., and achieve the effect of convenient mass production and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Preparation of anti-human kininogenase hybridoma cell line
[0054] 1. Animal immunization
[0055] BALB / c female mice (purchased from Changzhou Cavens Experimental Animal Co., Ltd.) were immunized with recombinant human kininogenase (expressed in Chinese hamster ovary cells, see patent 201310746269.X for the preparation method) according to the general immunization procedure. For specific immunization conditions, please refer to the "Experimental Guidelines for Antibody Preparation and Use". The serum titer of immunized mice was tracked by indirect ELISA method, and the immunized mouse with the highest serum titer was selected, and the fusion experiment of mouse splenocytes and mouse myeloma cells was carried out.
[0056] 2. Cell Fusion
[0057] (1). Preparation of spleen cells
[0058] Take the immunized mice, remove their eyeballs, take blood, put them to death by breaking the cervical spine, soak them in 75% (v / v) alcohol for 10 minutes, take out thei...
Embodiment 2
[0068] Example 2. Determination of the variable region sequence of the hybridoma cell line antibody
[0069] The variable region sequences of the above-mentioned hybridoma cell lines C24 and C32 antibodies were determined.
[0070] a. Extraction of RNA: Extract the total RNA of the above-mentioned hybridoma cell lines C24 and C32 with reference to the instructions of the Total Cell RNA Extraction Kit (purchased from Roche Company) and perform reverse transcription immediately;
[0071] b. Reverse transcription of RNA into DNA: Refer to Thermo Scientific Reverted First strand cDNA Synthesis Kit (purchased from Thermo Company) to reverse-transcribe the total RNA extracted in the previous step to obtain cDNA, and freeze it at -20°C for later use;
[0072]c. PCR amplification and recovery of the variable region sequence: the cDNA obtained in the above step is used as a template, and the variable region sequence of the heavy chain and light chain is sequenced with the general prime...
Embodiment 3
[0076] Example 3. Recombinant expression and purification of single-chain antibody
[0077] According to the sequencing results in Example 2, a connecting peptide (GGGGS) was added between the heavy chain and light chain variable regions of the hybridoma cell lines C24 and C32, respectively. 3 , introduce six histidines, and optimize the codons of the entire gene according to the preference of the Pichia pastoris expression system to perform recombinant expression of single-chain antibodies. The expressed antibodies were named Antibody A24 and Antibody A32 respectively, and their structures and compositions are shown in the attached figure 2 shown. The recombinant expression of the above-mentioned single-chain antibody has the following characteristics:
[0078] 1. Construction of expression plasmids for fusion protein genes
[0079] The gene sequence of antibody A24 after codon optimization is shown in SEQ ID NO:19, the amino acid sequence is shown in SEQ ID NO:17; the nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com