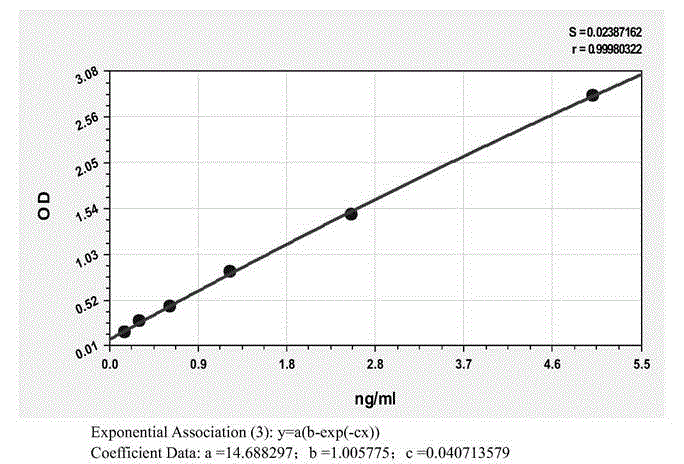
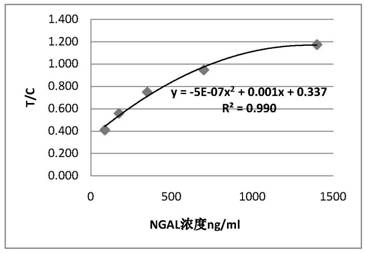
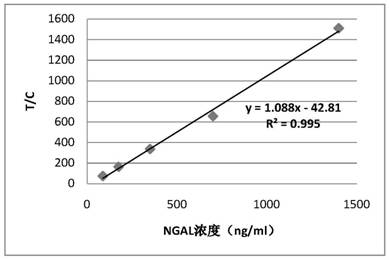
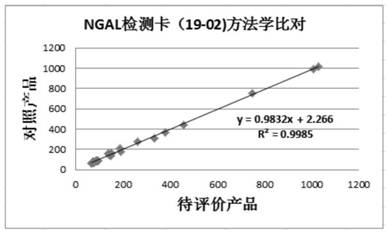
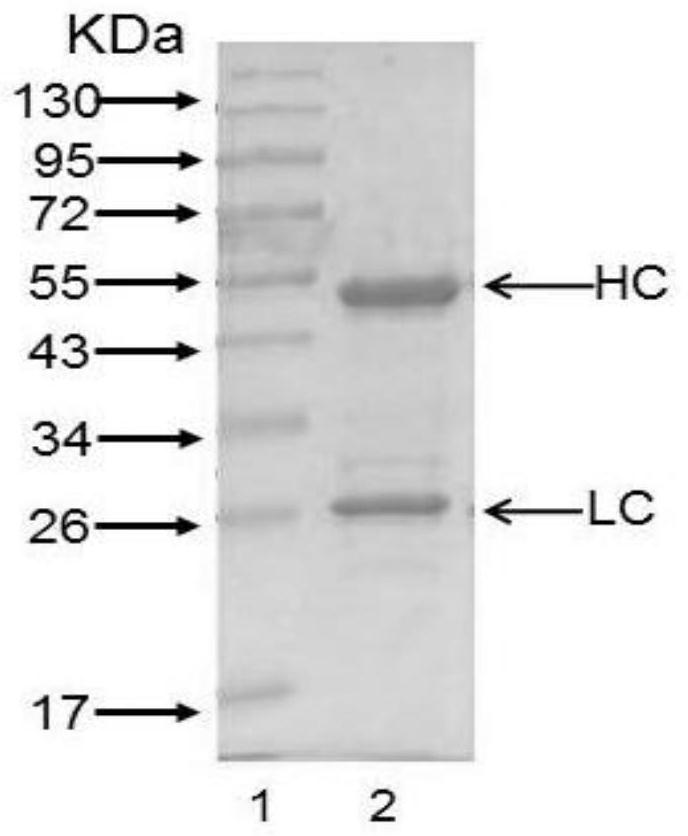
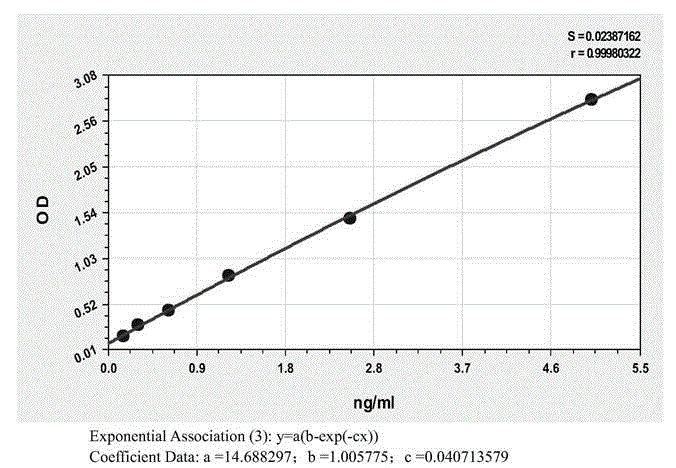
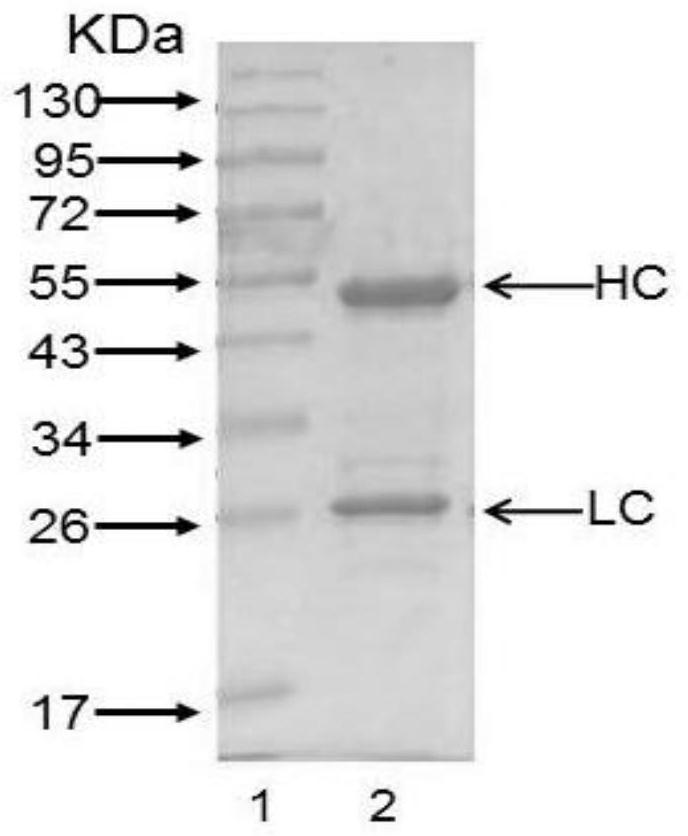
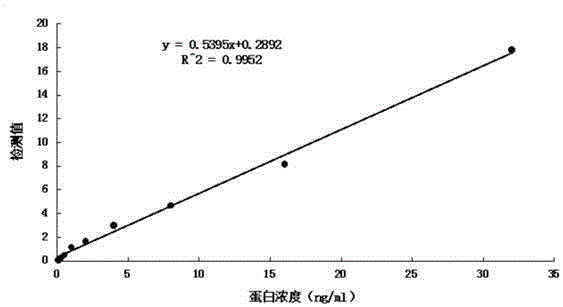
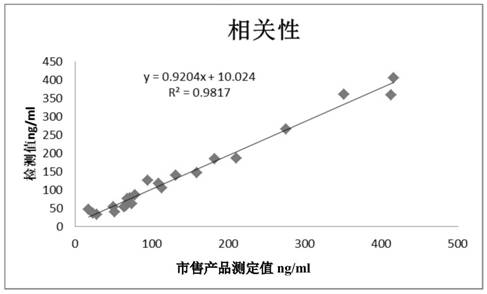
Patents
Literature
40results about How to "Meet the needs of large-scale clinical applications" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti-human-CPR (C reactive protein) antibody and application thereof
ActiveCN105713091AMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against animals/humansBiological testingImmunofluorescenceTrue positive rate
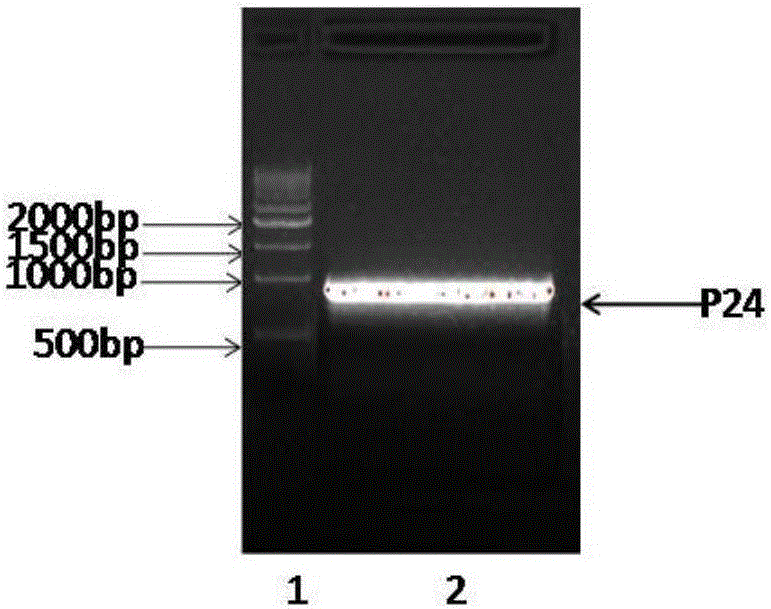
The invention relates to an anti-human-CPR (C reactive protein) antibody and an application thereof. Multiple antibodies are prepared and subjected to matched screening, and an antibody combination (P24 and P03) with sensitivity and specificity meeting the requirement is obtained; meanwhile, mass production of the antibody combination is facilitated, and the requirement of later large-scale clinical application can be met. The antibody combination is subjected to debugging optimization work by a detection system, a time-resolved immunofluorescence chromatography quantitative detection card for human CPR is obtained and is simple and convenient to operate, and the sensitivity, the specificity and related detection performance of the time-resolved immunofluorescence chromatography quantitative detection card can meet the requirement of human clinical sample detection.
Owner:ZONHON BIOPHARMA INST
Human procalcitonin colloidal gold quantitative detection card
ActiveCN106093431AMeet the needs of large-scale clinical applicationsEase of mass productionBiological testingImmunofluorescenceTrue positive rate
The present invention relates to anti-human procalcitonin antibody and applications thereof. According to the present invention, a variety of antibodies are prepared, and pairing screening is performed to obtain the antibody combination having sensitivity and specificity, wherein the sensitivity and the specificity can meet the requirements; mass production is convenient, and the large-scale clinical application requirements in the future can be met; and the debugging optimization work of the detection system is performed on the antibody combination to obtain the time-resolved immunofluorescence chromatography quantitative detection card of the human procalcitonin, wherein the operation of the detection card is convenient, and the sensitivity, the specificity and the related detection performance of the detection card can meet the human clinical sample detection.
Owner:江苏晶红生物医药科技股份有限公司
Anti-human C-reactive protein antibody and application thereof
ActiveCN106699884AEase of mass productionSimple and efficient operationImmunoglobulins against animals/humansBiological material analysisQuantitative determinationAntibody
The invention relates to anti-human C-reactive protein (CRP) antibody, a preparation method thereof and application of the antibody in anti-human C-reactive protein detection. An inventor prepares a variety of antibodies, and antibody combinations (P20 and P02) capable of meeting the sensitivity and specificity demands are obtained through pairing and screening. In addition, large-scale production of the antibodies is promoted, and the future large-scale clinic application demand can be met. A colloidal gold immunochromatography quantitative determination card simple and convenient to operate and having sensitivity, specificity and relevant detection properties capable of meeting human clinic sample detection, of a detected human C-reactive protein can be obtained through the detection system debugging and optimizing work to the antibody combinations.
Owner:ZONHON BIOPHARMA INST
Anti-human CD26 antibody and application of anti-human CD26 antibody in detection kit
ActiveCN109535255AMeet performance requirementsEase of mass productionImmunoglobulins against cell receptors/antigens/surface-determinantsNucleic acid vectorComplementarity determining regionHuman antimouse Antibody
The invention relates to an anti-human CD26 antibody, and preparation and a commercial application of the anti-human CD26 antibody, and belongs to the field of in vitro diagnosis. A heavy chain variable region of the anti-human CD26 antibody comprises the following complementarity-determining regions: HCDR1 with an amino acid sequence shown as SEQ ID NO: (sequence identifier number) 1, HCDR2 withan amino acid sequence shown as SEQ ID NO: 2, and HCDR3 with an amino acid sequence shown as SEQ ID NO: 3; and a light chain variable region of the anti-human CD26 antibody comprises the following complementarity-determining regions: LCDR1 with an amino acid sequence shown as SEQ ID NO: 4, LCDR2 with an amino acid sequence shown as SEQ ID NO: 5, and LCDR3 with an amino acid sequence shown as SEQ ID NO: 6. Relevant detection performance, such as specificity, of the anti-human CD26 antibody can meet performance requirements of an immunohistochemical kit; and a chimeric humanized antibody constructed by the applicant on a basis of the antibody is more convenient to recombine, express and produce massively, reduces a human anti-mouse antibody response and non-specific adsorption and can meet requirements of large-scale clinical application.
Owner:ZONHON BIOPHARMA INST
Fluorogenic quantitative detection test card for human C-reactive protein
ActiveCN105842440AEase of mass productionSimple and efficient operationMaterial analysisC-reactive proteinImmunology
The invention relates to anti-human C-reactive protein antibody and application thereof. The invention prepares a plurality of antibodies, and performs pairing and screening to obtain the antibody combination (P24 and P03) with which sensitivity and specificity can satisfy the requirement. The antibody is convenient to produce in large quantities, and can meet the requirement of clinical application in large-scale. The time-resolved fluoroimmunoassay chromatography quantitative detection card of human C-reactive protein having meeting clinical sample detection performances such as sensitivity, specificity and related detection performance is obtained by means of adjusting and optimizing of detection system for the antibody combination, and the detection card is easy and simple to operate.
Owner:江苏晶红生物医药科技股份有限公司
Anti-C-reactive-protein antibodies and application thereof
ActiveCN105949309AMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against animals/humansBiological testingImmunofluorescenceTrue positive rate
The invention relates to anti-C-reactive-protein antibodies and an application thereof. Various antibodies are prepared, and pairing and screening are carried out to obtain antibody combinations (P24 and P03), the sensitivity and specificity of which can meet requirements. Massive production of the antibodies becomes convenient, and the antibodies can meet requirements of clinical applications on a large scale. The antibody combinations are subjected to debugging and optimization of a detection system, and a time-resolved fluoroimmuno-chromatography quantitative detection card of C-reactive protein is obtained, wherein the card is easy to operate and has sensitivity, specificity and related detection performance which can meet detection of a human clinical sample.
Owner:ZONHON BIOPHARMA INST
Anti-human beta2-microglobulin antibody and application thereof
ActiveCN109666072AMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against animals/humansBiological testingMedicineBeta-2 microglobulin
The invention relates to a novel anti-human beta2-microglobulin antibody and application thereof, and belongs to the field of immunochemistry. Various antibodies are prepared, paired and screened, andthe antibody combination (BM12 and BM17) is obtained, wherein the sensitivity and the specificity of the antibody combination can meet requirements. Mass production is facilitated, and future requirements of large-scale clinical application can be met. The antibody combination is subjected to debugging and optimizing of a detection system, and industrial application of collaurum rapid test papercards of human beta2-microglobulin is obtained, wherein the collaurum rapid test paper cards are convenient to operate, and the sensitivity, the specificity and correlation detection performance of the collaurum rapid test paper cards can meet human clinical sample detection.
Owner:ZONHON BIOPHARMA INST
Quantitative detection card of human neutrophil gelatinase-associated lipocalin and clinical application thereof
ActiveCN109709340AMeet the needs of large-scale clinical applicationsEase of mass productionBiological testingBacteriuriaLipocalin
The invention relates to a quantitative detection card of human neutrophil gelatinase-associated lipocalin and clinical application thereof. Several kinds of antibodies are prepared and pairing and screening are carried out; and one group of antibodies including NG02 and NG19 that have the sensitivity and specificity meeting the requirements of the quantitative detection card is obtained. Meanwhile, the large-scale production is realized conveniently and thus the need of large-scale clinical application in future is met. After debugging optimization work of the detection system on the antibodygroup, the quantitative detection card of human neutrophil gelatinase-associated lipocalin is obtained, wherein the quantitative detection card has advantages of simple operation and high sensitivityand has the specificity and related detection performance that can meet the body blood and urine sample detection requirement.
Owner:ZONHON BIOPHARMA INST
Human beta2-microglobulin quantitative test card and clinical applications
ActiveCN109725159AMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against cell receptors/antigens/surface-determinantsBiological testingTest cardTrue positive rate
The present invention relates to a human beta2-microglobulin quantitative test card. The invention prepares a plurality of antibodies and performs paired screening to obtain a group of antibody combinations (BM12 and BM17) having sensitivity and specificity which can meet requirements of the quantitative test card. At the same time, the test card is convenient for mass production, and can meet theneeds of large-scale clinical applications in the future. The antibody combination is debugged and optimized in the detection system to obtain a colloidal gold immunochromatographic quantitative testcard for human beta2-microglobulin which is easy to operate, sensitive, specific and has related detection performance meeting the detection of human blood or urine samples.
Owner:ZONHON BIOPHARMA INST
Test paper card for fluorescent quantitative detection of human PCT (procalcitonin)
ActiveCN106046157AMeet the needs of large-scale clinical applicationsTime-resolvedImmunoglobulins against hormonesBiological testingFluorescenceImmunofluorescence
The invention relates to a test paper card for fluorescent quantitative detection of human PCT (procalcitonin). According to the invention, various antibodies are prepared and subjected to pairing screening, and an antibody combination with sensitivity and specificity both meeting requirements is obtained; mass production of the test paper card is facilitated, and the requirement for large-scale clinical application in the future can be met. The antibody combination is subjected to a debugging and optimizing work of a detection system, the time-resolved immunofluorescence chromatography quantitative detection card which is simple and convenient to operate and applied to the human PCT is obtained, and the sensitivity, the specificity and related detection performance of the card can meet the requirements of human clinical sample detection.
Owner:江苏晶红生物医药科技股份有限公司
ELISA quantitative detection kit for human tissue kallikrein 1
ActiveCN105987998AMeet the needs of large-scale clinical applicationsEase of mass productionMaterial analysisEnzymeKallikrein
The invention relates to an ELISA quantitative detection kit for detecting human tissue kallikrein 1 (hK1) and related antibodies. According to the invention, a plurality of monoclonal antibodies are prepared and are paired and screened, so an antibody combination (A24 and A32) with sensitivity and specificity meeting demands is obtained; and the antibody combination can be conveniently produced in a large scale and can meet demands of large-scale clinical application in the future. The antibody combination is subjected to adjustment and optimization of a detection system so as to obtain the ELISA quantitative detection kit for human tissue kallikrein 1 with the advantages of simple operation and good sensitivity, specificity and related detection performance.
Owner:ZONHON BIOPHARMA INST
Anti-human ngal antibody and its application in detection test paper card
ActiveCN109608542BMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against animals/humansBiological testingAntiendomysial antibodiesEngineering
The invention relates to an anti-human neutrophil gelatinase-associated lipocalin antibody and its application in detection test paper cards. The present invention prepares a variety of antibodies and conducts pair screening to obtain a group of antibody combinations (NG02 and NG19) that can meet the needs of sensitivity and specificity; meanwhile, it is convenient for mass production and can meet the needs of large-scale clinical application in the future. Debugging and optimizing the detection system of the above antibody combinations, and obtaining a colloidal gold immunoassay for human neutrophil gelatinase-associated lipocalin that is easy to operate, sensitive, specific and related detection performance can meet the detection of human blood or urine samples Chromatographic quantitative detection card.
Owner:ZONHON BIOPHARMA INST
Anti-human procalcitonin antibody and application thereof
ActiveCN105949311AMeet the needs of large-scale clinical applicationsEase of mass productionBiological material analysisImmunoglobulins against hormonesImmunofluorescenceAntiendomysial antibodies
The invention relates to an anti-human procalcitonin antibody and application thereof. A variety of antibodies are prepared, matched and screened, and an antibody combination with the sensitivity and specificity meeting requirements is obtained; meanwhile, mass production is convenient, and the requirement for large-scale clinic application in the future can be met. Debugging and optimization work of a detection system is carried out on the antibody combination, and a human PCT time-resolved immunofluorescence chromatography quantitative test card which is easy and convenient to operate is obtained, wherein the sensitivity, specificity and relevant detection performance can meet human clinic sample detection.
Owner:ZONHON BIOPHARMA INST
Anti-human myoglobin antibody and its application in detection kit
ActiveCN109721655BMeet the needs of large-scale clinical applicationsEase of mass productionBacteriaImmunoglobulins against animals/humansImmunochemistryMolecular biology
The invention relates to a novel anti-human myoglobin antibody and an application thereof in a detection kit, and belongs to the field of immunochemistry. According to the invention, a plurality of antibodies are prepared, and paired screening is carried out to obtain an antibody combination (M26 and M08) which meets requirements in sensitivity and specificity. Meanwhile, the antibody combinationis convenient for mass production and can meet the requirement of future large-scale clinical application. The debugging optimization work of a detection system for the antibody combination is carriedout to completely meet the application requirements of preparing fluorescent rapid detection test paper cards for human myoglobin. The fluorescent rapid detection test paper cards are simple and convenient to operate, and accord with commercial application indexes in sensitivity, specificity and related detection performance.
Owner:ZONHON BIOPHARMA INST
Beta2-microglobulin detection kit and clinical application thereof
ActiveCN113621059AHigh expressionShort cycleImmunoglobulins against animals/humansBiological material analysisAntigenBlood specimen
The invention relates to five anti-human beta2-microglobulin (beta2-MG) antibodies and application thereof in a detection kit for detecting the content of human beta2-microglobulin in a blood sample. Specifically, the invention provides five mouse anti-human beta2-MG monoclonal antibodies and an application of the mouse anti-human beta2-MG monoclonal antibodies in a latex enhanced immunoturbidimetry detection kit. According to the invention, a plurality of antibodies are prepared and are paired and screened to obtain an antibody combination with sensitivity and specificity meeting requirements; meanwhile, the recombinant human beta2-MG is adopted as an antigen calibrator, so that the recombinant human beta2-MG can be conveniently and stably produced in batches with controllable quality, and the requirements of large-scale clinical application in the future can be met. A latex immunoturbidimetric quantitative detection reagent which is simple and convenient to operate and has sensitivity, specificity and related detection performance meeting human clinical sample detection is obtained by debugging and optimizing a detection system of the antibody combination.
Owner:ZONHON BIOPHARMA INST
Human tissue kallikrein 1 fluorescent quantitative detection test paper card
ActiveCN105987997BMeet the needs of large-scale clinical applicationsEase of mass productionMaterial analysisImmunofluorescenceFluorescence
The invention relates to a fluorescent quantitative test strip for human tissue kallikrein 1 (hK1) and related antibodies. According to the invention, a plurality of monoclonal antibodies are prepared and are paired and screened, so an antibody combination (A24 and A32) with sensitivity and specificity meeting demands is obtained; and the antibody combination can be conveniently produced in a large scale and can meet demands of large-scale clinical application in the future. The antibody combination is subjected to adjustment and optimization of a detection system so as to obtain the time-resolved immunofluorescent chromatographic quantitative test strip for human tissue kallikrein 1 with the advantages of simple operation and good sensitivity, specificity and related detection performance.
Owner:ZONHON BIOPHARMA INST
Immunohistochemical detection kit for human CD26 and clinical application thereof
ActiveCN109709337APrecision therapyMeet performance requirementsBiological testingComplementarity determining regionHuman antimouse Antibody
The invention, which belongs to the field of in vitro diagnosis, relates to an immunohistochemical detection kit for human CD26 and clinical application thereof. According to the disclosed immunohistochemical detection kit, a novel anti-human-CD26 antibody is employed; the heavy chain variable region contains following complementarity determining regions: HCDR1, HCDR2, and HCDR3 having the amino acid sequences shown by SEQ ID NO:1, 2, and 3 respectively; and light chain variable region sequences contain following complementarity determining regions: LCDR1, LCDR2, LCDR3 having the amino acid sequences shown by SEQ ID NO:4, 5, 6. According to the invention, the performance requirement of the immunohistochemical kit can be met by related detection performances like the specificity of the anti-human-CD26 antibody; the antibody can be a mouse antibody or a recombinantly expressed chimeric human antibody and is convenient to product in a large scale; with the recombinantly expressed chimerichuman antibody, the human anti-mouse antibody response is reduced and the non-specific adsorption is reduced; and thus the need of large-scale clinical application can be met. In the application of the CD26 target drug, the effect of precise treatment is realized by using the immunohistochemical detection kit.
Owner:ZONHON BIOPHARMA INST
A kind of anti-human cd26 antibody and its application in detection kit
ActiveCN109535255BMeet performance requirementsEase of mass productionImmunoglobulins against cell receptors/antigens/surface-determinantsNucleic acid vectorComplementarity determining regionHeavy chain
The invention relates to an anti-human CD26 antibody, and preparation and a commercial application of the anti-human CD26 antibody, and belongs to the field of in vitro diagnosis. A heavy chain variable region of the anti-human CD26 antibody comprises the following complementarity-determining regions: HCDR1 with an amino acid sequence shown as SEQ ID NO: (sequence identifier number) 1, HCDR2 withan amino acid sequence shown as SEQ ID NO: 2, and HCDR3 with an amino acid sequence shown as SEQ ID NO: 3; and a light chain variable region of the anti-human CD26 antibody comprises the following complementarity-determining regions: LCDR1 with an amino acid sequence shown as SEQ ID NO: 4, LCDR2 with an amino acid sequence shown as SEQ ID NO: 5, and LCDR3 with an amino acid sequence shown as SEQ ID NO: 6. Relevant detection performance, such as specificity, of the anti-human CD26 antibody can meet performance requirements of an immunohistochemical kit; and a chimeric humanized antibody constructed by the applicant on a basis of the antibody is more convenient to recombine, express and produce massively, reduces a human anti-mouse antibody response and non-specific adsorption and can meet requirements of large-scale clinical application.
Owner:ZONHON BIOPHARMA INST
.Human c-reactive protein colloidal gold quantitative detection card
ActiveCN106596976BMeet the needs of large-scale clinical applicationsEase of mass productionBiological material analysisImmunoglobulinsTrue positive rateBioinformatics
The invention relates to a human C-reactive protein colloidal gold quantitative detection card, two anti-human C-reactive protein (CRP) antibodies adopted for the card, and a preparation method of the antibodies. Multiple antibodies are prepared by an inventor, paired and screened, and antibody combinations (P20 and P02) with sensitivity and specificity both meeting requirements are obtained. Mass production is convenient, and the requirements of large-scale clinical application in future can be met. The antibody combinations are subjected to debugging optimization work of a detection system, the human C-reactive protein colloidal gold immunochromatography quantitative detection card which is easy and convenient to operate and has sensitivity, specificity and relevant detection performance greatly improved is obtained, and the clinical sample detection requirements are completely met.
Owner:江苏晶红生物医药科技股份有限公司
Anti-human tissue kallikrein 1 antibody and application thereof
ActiveCN105985437AMeet the needs of large-scale clinical applicationsEase of mass productionFermentationGenetic engineeringImmunofluorescenceKinin
The invention relates to an anti-human tissue kallikrein 1 antibody and an application thereof. The invention provides a plurality of monoclonal antibodies. Through matching screening, an antibody combination (A24 and A32) with sensitivity and specificity meeting certain demand is obtained. The antibody combination is convenient for mass production, such that the need of large-scale clinical application in future can be satisfied. The antibody combination is subjected to detection system debugging optimization, such that a human tissue kallikrein 1 enzyme-linked immune quantitative detection kit, a human tissue kallikrein 1 colloidal gold immunochromatographic quantitative detection card and a human tissue kallikrein 1 time-resolved immunofluorescence chromatographic quantitative detection card, which are simple to operate and the sensitivity, selectivity and related detection performances of which can satisfy human plasma sample detection, can be obtained.
Owner:ZONHON BIOPHARMA INST
Anti-human procalcitonin antibody and its application
ActiveCN105949311BMeet the needs of large-scale clinical applicationsEase of mass productionBiological material analysisImmunoglobulins against hormonesImmunofluorescenceTrue positive rate
The invention relates to an anti-human procalcitonin antibody and application thereof. A variety of antibodies are prepared, matched and screened, and an antibody combination with the sensitivity and specificity meeting requirements is obtained; meanwhile, mass production is convenient, and the requirement for large-scale clinic application in the future can be met. Debugging and optimization work of a detection system is carried out on the antibody combination, and a human PCT time-resolved immunofluorescence chromatography quantitative test card which is easy and convenient to operate is obtained, wherein the sensitivity, specificity and relevant detection performance can meet human clinic sample detection.
Owner:ZONHON BIOPHARMA INST
Anti-human tissue kallikrein 1 antibody and its application
ActiveCN105985437BMeet the needs of large-scale clinical applicationsEase of mass productionGenetic engineeringFermentationKininBlood plasma
The invention relates to an anti-human tissue kallikrein 1 antibody and an application thereof. The invention provides a plurality of monoclonal antibodies. Through matching screening, an antibody combination (A24 and A32) with sensitivity and specificity meeting certain demand is obtained. The antibody combination is convenient for mass production, such that the need of large-scale clinical application in future can be satisfied. The antibody combination is subjected to detection system debugging optimization, such that a human tissue kallikrein 1 enzyme-linked immune quantitative detection kit, a human tissue kallikrein 1 colloidal gold immunochromatographic quantitative detection card and a human tissue kallikrein 1 time-resolved immunofluorescence chromatographic quantitative detection card, which are simple to operate and the sensitivity, selectivity and related detection performances of which can satisfy human plasma sample detection, can be obtained.
Owner:ZONHON BIOPHARMA INST
Colloidal gold quantitative detection test card for human tissue kallikrein 1
ActiveCN105988004BMeet the needs of large-scale clinical applicationsEase of mass productionBiological testingImmunoglobulins against enzymesTrue positive rateMonoclonal antibody
The invention relates to a colloidal gold quantitative test strip for human tissue kallikrein 1 (hK1) and related antibodies. According to the invention, a plurality of monoclonal antibodies are prepared and are paired and screened, so an antibody combination (A24 and A32) with sensitivity and specificity meeting demands is obtained; and the antibody combination can be conveniently produced in a large scale and can meet demands of large-scale clinical application in the future. The antibody combination is subjected to adjustment and optimization of a detection system so as to obtain the colloidal gold immunochromatographic quantitative test strip for human tissue kallikrein 1 with the advantages of simple operation and good sensitivity, specificity and related detection performance.
Owner:ZONHON BIOPHARMA INST
Human Procalcitonin Colloidal Gold Quantitative Detection Card
ActiveCN106093431BMeet the needs of large-scale clinical applicationsEase of mass productionBiological testingImmunofluorescenceTrue positive rate
The present invention relates to anti-human procalcitonin antibody and applications thereof. According to the present invention, a variety of antibodies are prepared, and pairing screening is performed to obtain the antibody combination having sensitivity and specificity, wherein the sensitivity and the specificity can meet the requirements; mass production is convenient, and the large-scale clinical application requirements in the future can be met; and the debugging optimization work of the detection system is performed on the antibody combination to obtain the time-resolved immunofluorescence chromatography quantitative detection card of the human procalcitonin, wherein the operation of the detection card is convenient, and the sensitivity, the specificity and the related detection performance of the detection card can meet the human clinical sample detection.
Owner:江苏晶红生物医药科技股份有限公司
Human c-reactive protein fluorescent quantitative detection test card
ActiveCN105842440BMeet the needs of large-scale clinical applicationsEase of mass productionMaterial analysisImmunofluorescenceFluorescence
The invention relates to anti-human C-reactive protein antibody and application thereof. The invention prepares a plurality of antibodies, and performs pairing and screening to obtain the antibody combination (P24 and P03) with which sensitivity and specificity can satisfy the requirement. The antibody is convenient to produce in large quantities, and can meet the requirement of clinical application in large-scale. The time-resolved fluoroimmunoassay chromatography quantitative detection card of human C-reactive protein having meeting clinical sample detection performances such as sensitivity, specificity and related detection performance is obtained by means of adjusting and optimizing of detection system for the antibody combination, and the detection card is easy and simple to operate.
Owner:江苏晶红生物医药科技股份有限公司
Immunohistochemical detection kit for human cd26 and its clinical application
ActiveCN109709337BPrecision therapyMeet performance requirementsBiological testingHuman antimouse AntibodyComplementarity determining region
The invention relates to an immunohistochemical detection kit for human CD26 and its clinical application, belonging to the field of in vitro diagnosis. The immunohistochemical detection kit disclosed in the present invention adopts a brand-new anti-human CD26 antibody, and its heavy chain variable region contains the following complementarity determining regions: the amino acid sequences are as shown in SEQ ID NO: 1, 2, 3 respectively HCDR1, HCDR2, HCDR3; and their light chain variable region sequences contain the following complementarity determining regions: LCDR1, LCDR2, LCDR3 shown in SEQ ID NO: 4, 5, 6, respectively. The specificity of the anti-human CD26 antibody used in the present invention can meet the performance requirements of the immunohistochemical kit, and it can be a mouse antibody or a recombinantly expressed chimeric human antibody, which is convenient for mass production, wherein the recombinantly expressed chimeric antibody Combined with human antibodies to reduce human anti-mouse antibody reactions and non-specific adsorption, it can also meet the needs of large-scale clinical applications. When using the CD26 target drug, the immunohistochemical kit of the present invention can be used together to achieve the effect of precise treatment.
Owner:ZONHON BIOPHARMA INST
Human pct fluorescence quantitative detection test card
ActiveCN106046157BMeet the needs of large-scale clinical applicationsTime-resolvedImmunoglobulins against hormonesBiological testingImmunofluorescenceCT - Calcitonin
The invention relates to a test paper card for fluorescent quantitative detection of human PCT (procalcitonin). According to the invention, various antibodies are prepared and subjected to pairing screening, and an antibody combination with sensitivity and specificity both meeting requirements is obtained; mass production of the test paper card is facilitated, and the requirement for large-scale clinical application in the future can be met. The antibody combination is subjected to a debugging and optimizing work of a detection system, the time-resolved immunofluorescence chromatography quantitative detection card which is simple and convenient to operate and applied to the human PCT is obtained, and the sensitivity, the specificity and related detection performance of the card can meet the requirements of human clinical sample detection.
Owner:江苏晶红生物医药科技股份有限公司
Human Tissue Kallikrein 1 Elisa Quantitative Detection Kit
ActiveCN105987998BMeet the needs of large-scale clinical applicationsEase of mass productionMaterial analysisMonoclonal antibodyTrue positive rate
The invention relates to an ELISA quantitative detection kit for detecting human tissue kallikrein 1 (hK1) and related antibodies. According to the invention, a plurality of monoclonal antibodies are prepared and are paired and screened, so an antibody combination (A24 and A32) with sensitivity and specificity meeting demands is obtained; and the antibody combination can be conveniently produced in a large scale and can meet demands of large-scale clinical application in the future. The antibody combination is subjected to adjustment and optimization of a detection system so as to obtain the ELISA quantitative detection kit for human tissue kallikrein 1 with the advantages of simple operation and good sensitivity, specificity and related detection performance.
Owner:ZONHON BIOPHARMA INST
Anti-human myo antibody and its application in detection kit
ActiveCN109734803BMeet the needs of large-scale clinical applicationsEase of mass productionBacteriaImmunoglobulins against animals/humansAntiendomysial antibodiesImmunochemistry
The invention relates to a novel anti-human MYO antibody and its application in a detection kit, belonging to the field of immunochemistry. The present invention prepares a variety of antibodies, and performs paired screening to obtain antibody combinations (M26 and M08) that can meet the needs of sensitivity and specificity; meanwhile, it is convenient for mass production and can meet the needs of large-scale clinical applications in the future. The debugging and optimization of the detection system for the above-mentioned antibody combination can fully meet the application requirements of the preparation of the fluorescent rapid detection test paper card for human myoglobin. Indicators for commercial application.
Owner:ZONHON BIOPHARMA INST
Anti-human crp antibody and its application
ActiveCN105713091BMeet the needs of large-scale clinical applicationsEase of mass productionImmunoglobulins against animals/humansBiological testingImmunofluorescenceTrue positive rate
The invention relates to an anti-human-CPR (C reactive protein) antibody and an application thereof. Multiple antibodies are prepared and subjected to matched screening, and an antibody combination (P24 and P03) with sensitivity and specificity meeting the requirement is obtained; meanwhile, mass production of the antibody combination is facilitated, and the requirement of later large-scale clinical application can be met. The antibody combination is subjected to debugging optimization work by a detection system, a time-resolved immunofluorescence chromatography quantitative detection card for human CPR is obtained and is simple and convenient to operate, and the sensitivity, the specificity and related detection performance of the time-resolved immunofluorescence chromatography quantitative detection card can meet the requirement of human clinical sample detection.
Owner:ZONHON BIOPHARMA INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com