Method for producing pramlintide (PA) by virtue of bombyx mori bioreactor
A technology of Pramlintide and Bombyx mori, which is applied in the field of genetic engineering, can solve the problems of small molecular weight of Pramlintide and the difficulty of high-efficiency soluble expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
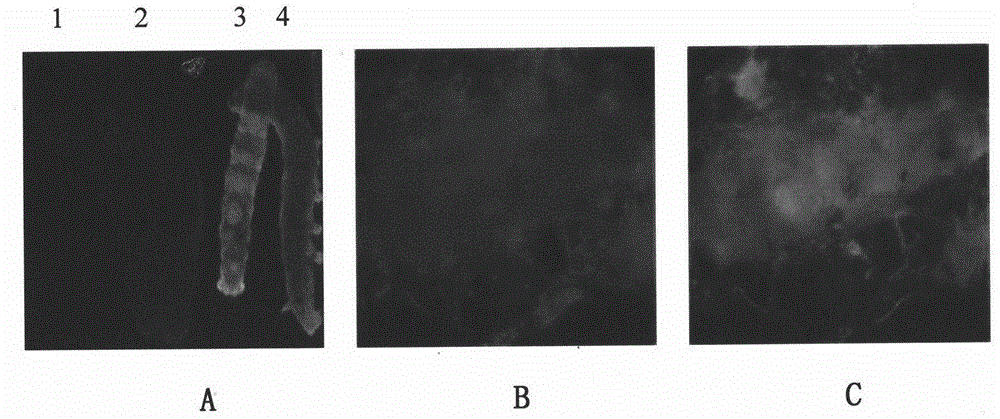
[0039] Example 1: Recombinant engineering bacteria E.coli SW106-BmNPV-PAM-PA virus infection of silkworm cell BmN expression protein
[0040] The constructed transfer vectors pFBDM-PAM-IM and pUCDM-PA-IG were introduced into the asd auxotrophic host Escherichia coli SW106 by transformation. During the transformation process, the transfer vectors were transposed, and the target gene fragments were integrated into the viral genome. Constructed into the viral genome BmMultiBacNPV-PAM-IM-PA-IG, the recombinant genetic engineering bacteria E.coli SW106 containing the viral genome BmMulitBacNPV-PAM-IM-PA-IG, in LB-Kan-Tet-Spe-Gm-Cm- Cultivate overnight on DAP solid medium, pick a single colony and inoculate in LB-Kan-Tet-Spe-Gm-Cm-DAP liquid medium for 10-12h, and the OD value reaches 0.8, take 1ml of the bacterial solution into a 1.5ml EP tube, Centrifuge at 3000rpm for 3min, discard the supernatant, resuspend the bacterial solution in sterilized water, centrifuge at 3000rpm for 3m...
Embodiment 2
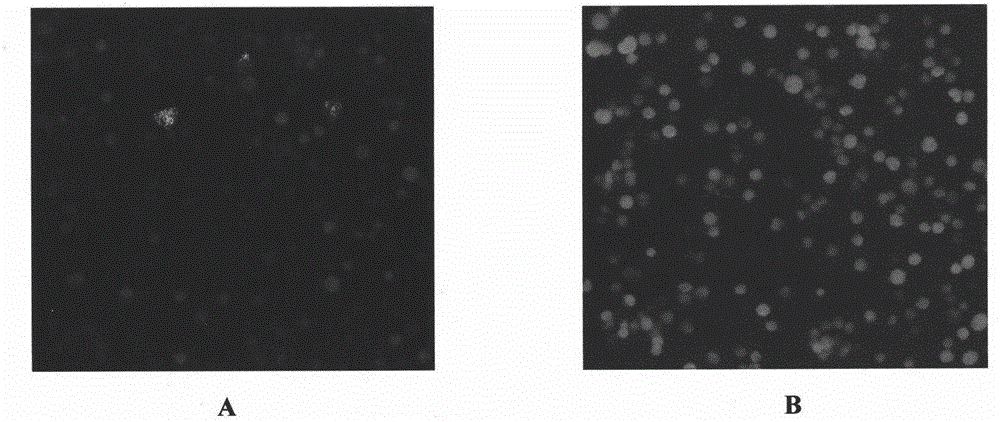
[0041] Example 2: Bombyx mori larvae were injected with recombinant baculovirus BmNPV-PAM-IM-PA-IG.
[0042] After the successful construction of the recombinant baculovirus BmNPV-PAM-IM-PA-IG, BmN cells were used for blind transmission. After 3 to 4 generations of blind passage, the cell Grace medium was collected directly, centrifuged at 12,000 rpm for 2 minutes, the supernatant was taken, and injected into silkworm larvae (from the 5th instar) at 10 μl / head. After 3 days, hemolymph of silkworm larvae was collected to observe whether red and green fluorescence appeared.
Embodiment 3
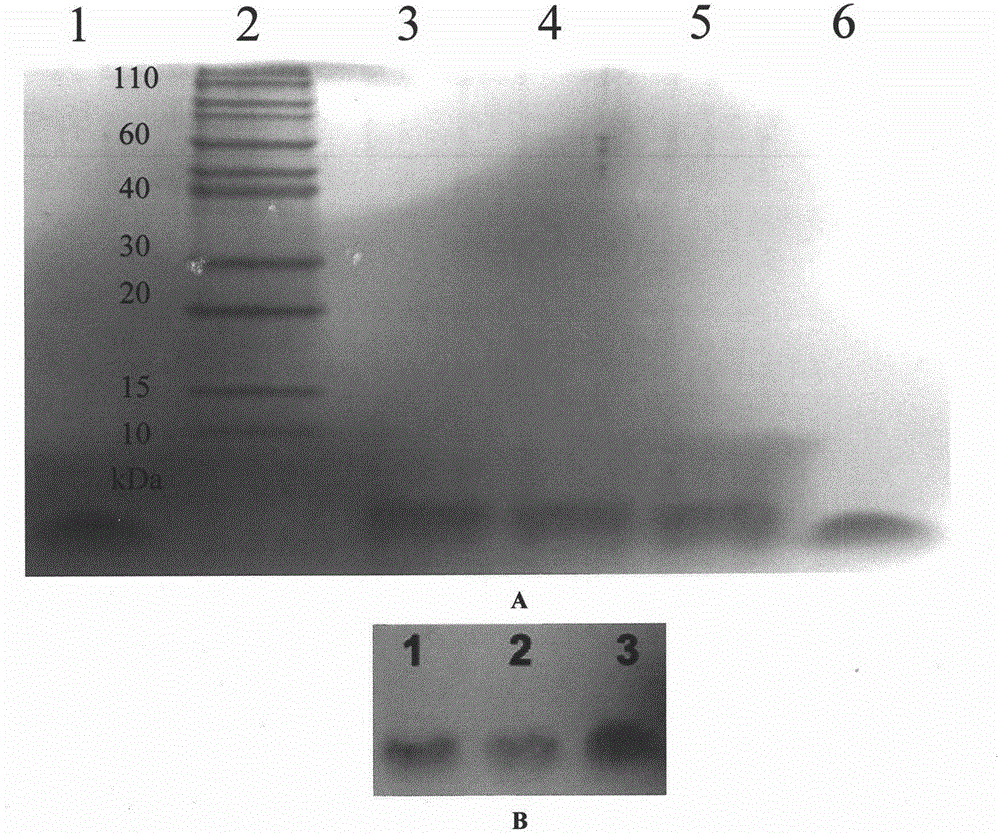
[0043] Embodiment 3: Purification of recombinant pramlintide
[0044] Collect silkworm larva hemolymph, add PMSF, centrifuge at 15000rpm for 10 minutes, take supernatant, add 3 times volume of PB solution (ph 6.0). Equilibrate the Ni-NTA affinity column with 20mM PB, 200mM NaCl (ph 6.0) solution 3ml / min, inject the expression supernatant into the Ni-NTA affinity column at 1ml / min, and elute with 20mM PB, 200mM NaCl, 20mM imidazole Miscellaneous protein, 20mM PB, 200mM NaCl, 150mM imidazole eluted protein. The flow-through protein was collected, the impurity protein was eluted with 20mM imidazole, and the target protein was eluted with 150mM imidazole, and the purified product was detected by SDS-PAGE gel and Western Blot.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com