4-((2-hydroxyethyl)(methyl)amino)-2-butanol and preparation method thereof
A technology of methylaminoethanol and hydroxyethyl, which is applied in the field of 4-amino)-2-butanol and its preparation, can solve problems such as weak capture ability, achieve fast absorption rate, large absorption capacity and simple preparation method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Synthesis method of 4-((2-hydroxyethyl)(methyl)amino)-2-butanol (HEMAB)
[0022]
[0023] Under ice bath conditions, 75.11g (1mol) of 2-methylaminoethanol was added dropwise to 70g (1mol) of butenone; after the addition, the stirring was continued for 1h. The reaction was stirred at room temperature (20-30°C) for 6 hours. The mixture was placed in an ice bath again, diluted with 30 mL of methanol, 37.9 g (1 mol) of sodium borohydride was added, and stirring was continued for 1 h; then the reaction was stirred at room temperature for 6 h. Under ice bath conditions, slowly add 30 mL of water to the mixture and continue to stir for 2 h. The obtained product was filtered to remove the sodium borohydride in the product; the obtained product was subjected to vacuum distillation at a temperature of 133-135°C and a condition of 20-22 inHg to obtain 4.55 g of the final product 4-((2-hydroxyl Ethyl)(methyl)amino)-2-butanol, the yield was 57.48%. The 4-((2-hydroxyethyl)...
Embodiment 2
[0024] Example 2: CO 2 Determination of absorption capacity
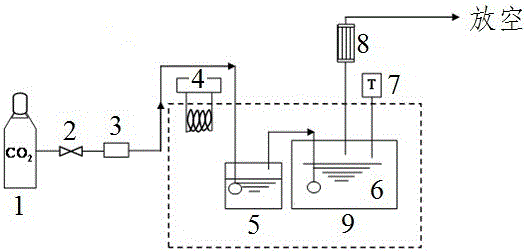
[0025] 4-((2-Hydroxyethyl)(methyl)amino)-2-butanol (HEMAB) is formulated into an aqueous solution with a concentration of 2mol / L as a carbon dioxide absorption liquid, and the CO is controlled under the condition of normal pressure of 0.1MPa 2 The volume fraction is 15% (ie CO 2 The partial pressure is 15kPa), the absorption equilibrium is reached within 8-10 hours, and the absorption capacity of HEMAB is measured. The test device belongs to the prior art, and its structure is as figure 1 Shown.
Embodiment 3
[0026] Example 3: CO in the gas to be absorbed 2 Partial pressure investigation
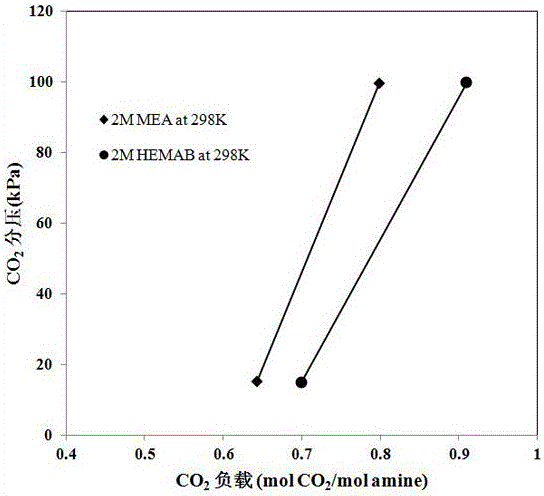
[0027] 4-((2-Hydroxyethyl)(methyl)amino)-2-butanol was formulated into an aqueous solution with a concentration of 2mol / L as a carbon dioxide absorption liquid, and the temperature of the absorbent was controlled to 25°C and 0.1MPa at normal pressure Next, measure the CO of HEMAB 2 Absorption capacity and CO 2 The relationship between the partial pressure and the CO of MEA under the same conditions 2 Comparison of absorption capacity, such as figure 2 Shown. figure 2 Is the CO of the aqueous solution of 4-((2-hydroxyethyl)(methyl)amino)-2-butanol under different partial pressures 2 Absorption capacity and CO of MEA aqueous solution 2 Comparison of absorption capacity, ◆ is MEA, ● is HEMAB.
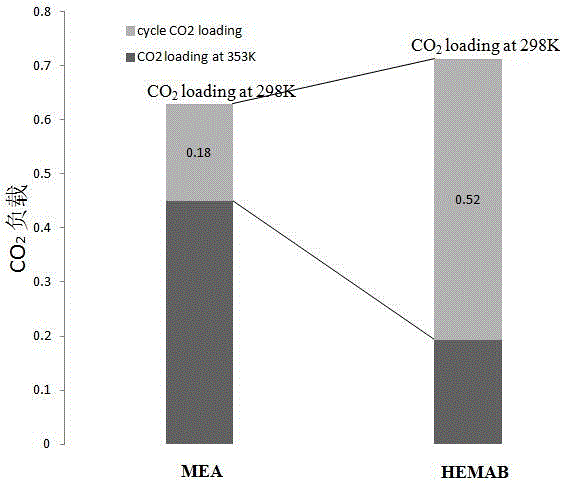
[0028] by figure 2 It can be seen that HEMAB absorbent can be applied to a wide range of CO 2 Volume fraction (0.5%-99% is acceptable, preferably 5%-60%), and in different CO 2 Under partial pressure, it has a l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com