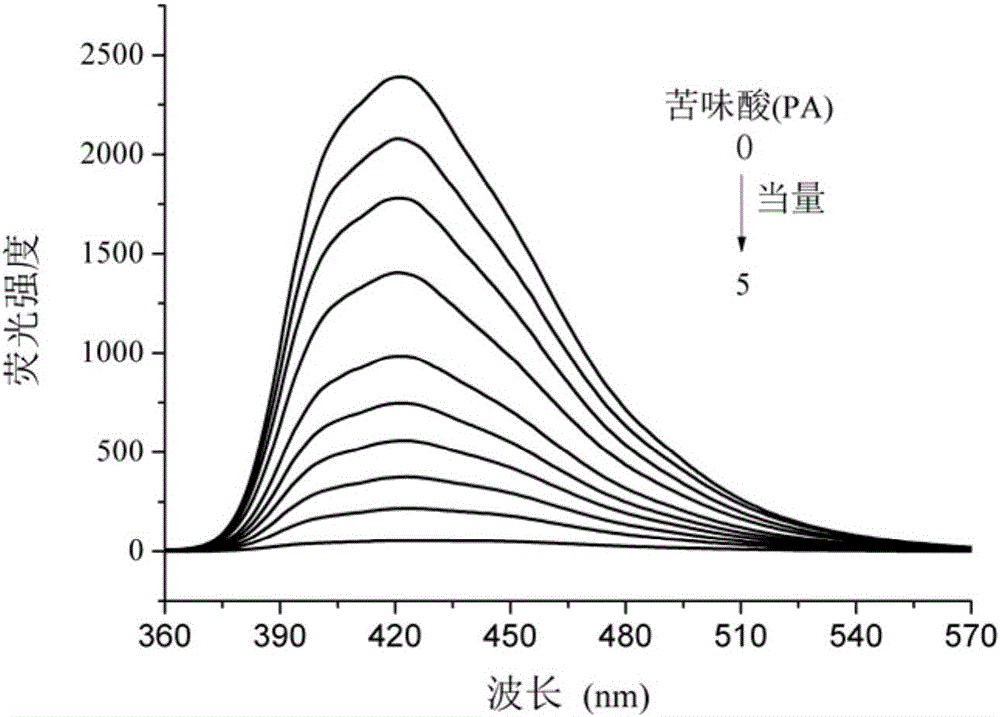
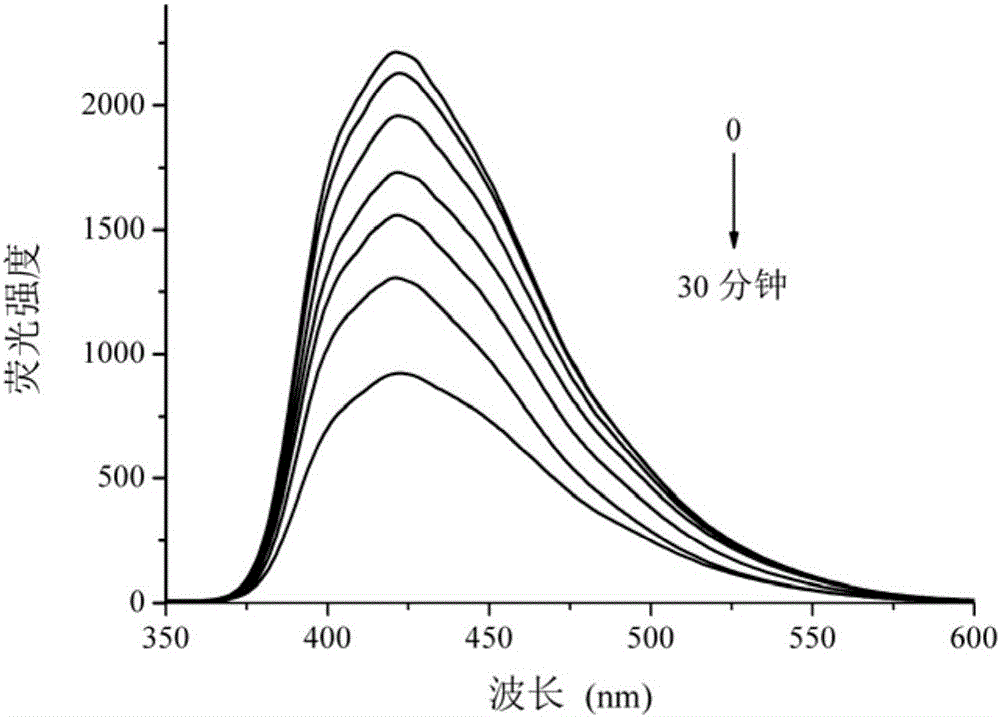
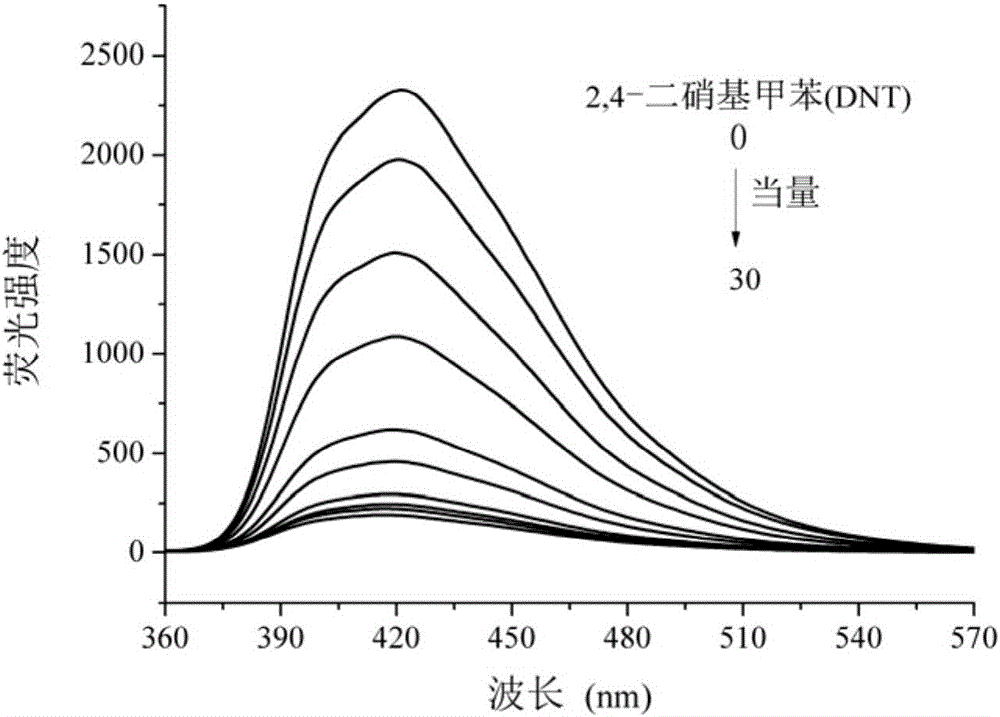
Benzimidazolyl chemical sensor used for fluorescence quenching detection of nitroaromatic explosives, and preparation method of benzimidazolyl chemical sensor
A benzimidazole-based, chemical sensor technology, applied in the field of detection of nitroaromatic explosives, can solve the problems of few types and difficult preparation of chemical sensors, and achieve the effects of easy temperature control, easy product, and rapid synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) With 0.523 grams of 1-bromododecane and 0.389 grams of 2,6-two (5-methylbenzimidazole) naphthalene (that is, in the structural formula of the aforementioned two (benzimidazole) naphthalene compounds, the benzimidazole group is located at The 2,6-position of the naphthalene ring, and R 1 =CH 3) as a raw material, according to the amount of substance ratio n (1-bromododecane):n (2,6-bis (5-methylbenzimidazole) naphthalene) = 2.1:1 mixed evenly, at a temperature of 80 ° C, 0.160 gram of NaOH solid is catalyst and 10 milliliters of acetonitrile is under the condition of solvent, carries out N-alkylation substitution reaction 2 hours;
[0042] (2) After the reaction is over, dissolve and transfer with ethyl acetate, wash the organic phase 3 times, take the organic phase, dry over anhydrous magnesium sulfate, spin off the solvent, and the crude product is separated by column chromatography to obtain the white solid bis(benzimidazole ) Naphthalene-based novel fluorescent...
Embodiment 2
[0049] (1) With 0.523 grams of 1-bromododecane and 0.389 grams of 2,6-two (5-methylbenzimidazole) naphthalene (that is, in the structural formula of the aforementioned two (benzimidazole) naphthalene compounds, the benzimidazole group is located at The 2,6-position of the naphthalene ring, and R 1 =CH 3 ) as a raw material, according to the amount of substance ratio n (1-bromododecane):n (2,6-bis (5-methylbenzimidazole) naphthalene) = 2.1:1 mixed evenly, at a temperature of 80 ° C, 0.080 gram of NaOH solid is catalyst and 10 milliliters of acetonitrile is under the condition of solvent, carries out N-alkylation substitution reaction 10 hours;
[0050] (2) After the reaction is over, dissolve and transfer with ethyl acetate, wash the organic phase 3 times, take the organic phase, dry over anhydrous magnesium sulfate, spin off the solvent, and the crude product is separated by column chromatography to obtain the white solid bis(benzimidazole ) Naphthalene-based novel fluoresce...
Embodiment 3
[0054] (1) With 1.247 grams of 1-bromododecane and 0.389 grams of 2,6-two (5-methylbenzimidazole) naphthalene (that is, in the structural formula of the aforementioned two (benzimidazole) naphthalene compounds, the benzimidazole group is located at The 2,6-position of the naphthalene ring, and R 1 =CH 3 ) as a raw material, according to the amount of substance ratio n (1-bromododecane):n (2,6-bis (5-methylbenzimidazole) naphthalene) = 5:1 mixed evenly, at a temperature of 80 ° C, 0.160 gram of NaOH solid is catalyst and 10 milliliters of acetonitrile is under the condition of solvent, carries out N-alkylation substitution reaction 2 hours;
[0055] (2) After the reaction is over, dissolve and transfer with ethyl acetate, wash the organic phase 3 times, take the organic phase, dry over anhydrous magnesium sulfate, spin off the solvent, and the crude product is separated by column chromatography to obtain the white solid bis(benzimidazole ) Naphthalene-based novel fluorescent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com