Docetaxel and berbamine double-load drug chitosan nanoparticles and preparation method thereof
A chitosan nanoparticle and docetaxel technology, which is applied in the directions of pharmaceutical formulations, antitumor drugs, and drug combinations, can solve the problems of poor absorption and low solubility of docetaxel, and achieves increased drug absorption, convenient use, and improved drug absorption. The effect of oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This embodiment relates to a preparation method of docetaxel-nicotinamide co-crystal, comprising the following steps:
[0055]Add 5 mg of docetaxel and nicotinamide (molar ratio 1:1) into 2.5 mL of methanol, and stir at 50° C. at 200 rpm for 2 h. The methanol solvent system is cooled to room temperature in a fume hood, the solvent in the system is slowly volatilized to precipitate crystals, and then dried in a vacuum desiccator.
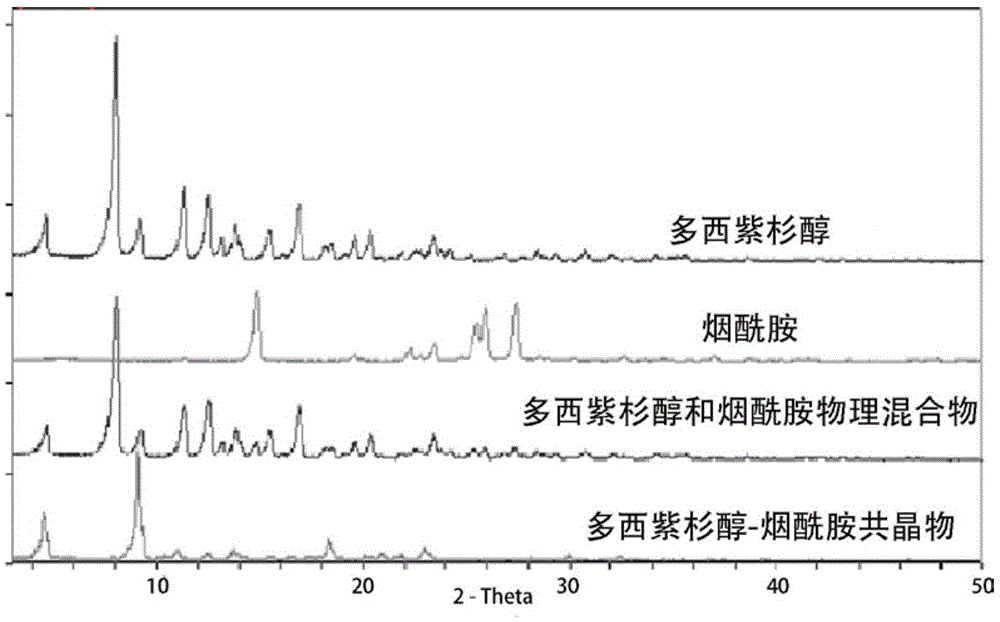
[0056] In the present embodiment, the XRD characterization of the docetaxel-nicotinamide cocrystal is as follows figure 1 shown by figure 1 It can be seen that both docetaxel and nicotinamide have obvious crystal diffraction peaks; in the spectrum of the physical mixture, the characteristic diffraction peaks of docetaxel are relatively obvious, and the characteristic peaks of nicotinamide are weakened, but there is no disappearance of the characteristic peaks; In the spectrum of the compound, the characteristic diffraction peaks of docetaxel...
Embodiment 2
[0058] This embodiment relates to a preparation method of docetaxel-nicotinamide co-crystal, comprising the following steps:
[0059] Add 5 mg of docetaxel and nicotinamide (molar ratio 1:10) into 5 mL of methanol, and stir at 60° C. at 500 rpm for 2 h. The methanol solvent system is cooled to room temperature in a fume hood, the solvent in the system is slowly volatilized to precipitate crystals, and then dried in a vacuum desiccator.
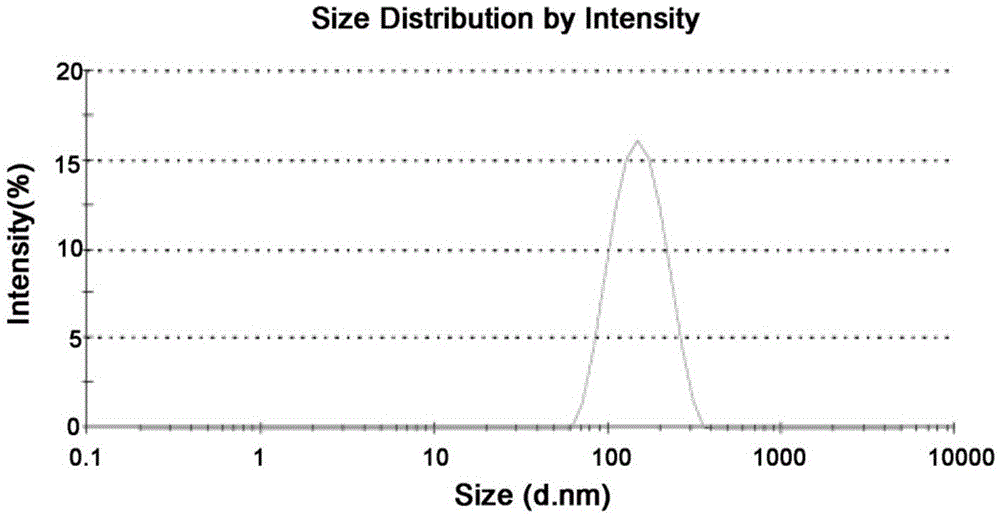
[0060] In this embodiment, the solubility of the docetaxel-nicotinamide co-crystal in water is 31 μg / mL.
Embodiment 3
[0062] This embodiment relates to a preparation method of docetaxel-nicotinamide co-crystal, comprising the following steps:
[0063] Add 5 mg of docetaxel and nicotinamide (molar ratio 1:20) into 20 mL of ethanol, and stir at 1500 rpm at 40° C. for 24 h. The ethanol solvent system is cooled to room temperature in a fume hood, the solvent in the system is slowly volatilized to precipitate crystals, and then dried in a vacuum desiccator. The docetaxel-nicotinamide co-crystal formation rate in this example was 97.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com