Iron-based catalyst for low-carbon olefin production through CO2 hydrogenation, and preparation and applications thereof
A technology for iron-based catalysts and low-carbon olefins, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, and hydrocarbon production from carbon oxides, etc., can solve the problem of wide distribution of hydrocarbon products and cost of preparation high catalyst particle size distribution, etc., to achieve the effect of low catalyst cost, simple preparation method and high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 15.81g FeCl 3 ·6H 2 O, 6.27g FeCl 2 4H 2 O, by Fe 3+ :Fe 2+ =65:35 molar ratio is mixed into the salt solution that Fe concentration is about 1mol / L, and the 12.1mol / L HCl solution of 2.5mL is added. Add 180 mL of 1.5 mol / L NaOH solution at a constant speed at 60°C under stirring conditions. Within about 1.5 h, the pH of the solution was adjusted from acidic to about 10.0. After the dropwise addition, keep the temperature and continue stirring for 1 h, and finally cool to room temperature. After the reaction, the deposited product was separated by magnetic field adsorption, washed fully with deionized water, and then dried at 60°C to obtain a catalyst sample. The sample was ground, pressed into tablets and passed through a 20-40 mesh sieve for later use. Fe 3 o 4 The synthesis reaction equation of is:
[0044] Fe 2+ +2Fe 3+ +8OH – →Fe(OH) 2 +2Fe(OH) 3 → Fe 3 o 4 +4H 2 O.
[0045] Reduction conditions: under normal pressure, pure H 2 , the temperature...
Embodiment 2
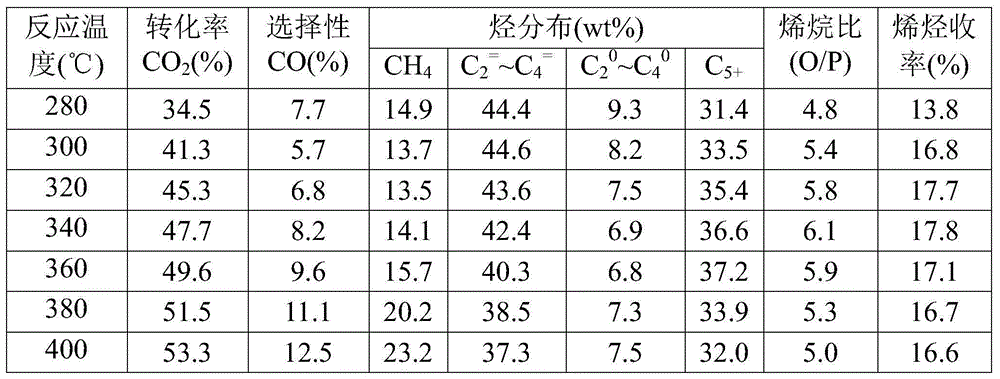
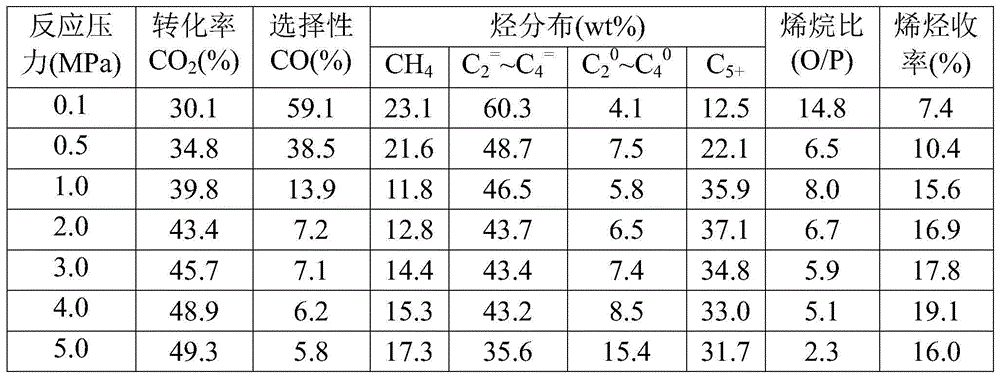
[0047] Take by weighing the catalyst sample 1.0g that embodiment 1 method prepares, evaluate in fixed-bed reactor: reduction condition: under normal pressure, pure H 2 , the temperature is 350°C, and the space velocity is 1500mL / (h·g cat ), the reduction time is 12h. Reaction condition: H 2 / CO 2 =3.0, the temperature is 320°C, the pressure is 0.1-5.0MPa, and the space velocity is 2000mL / (h·g cat ), the influence of the reaction pressure on the performance of the catalyst was investigated, and the test results (see Table 2) showed that as the reaction pressure increased, the CO 2 The conversion rate increased gradually, while the CO selectivity and C 2 = ~C 4 = Selectivity gradually decreases.
Embodiment 3
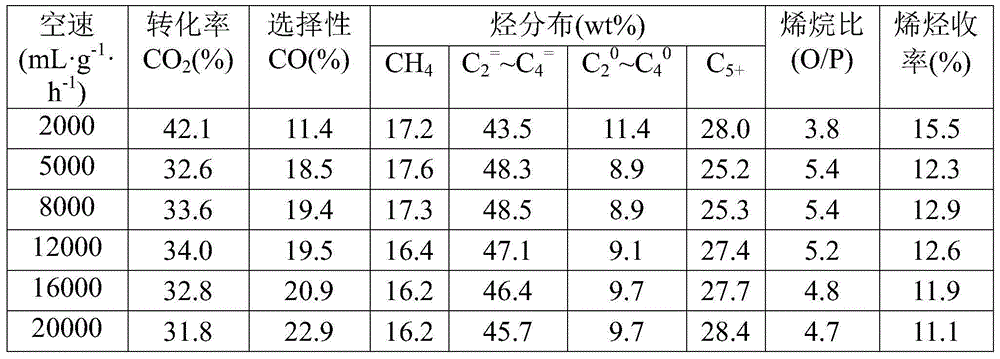
[0049] Take by weighing the catalyst sample 0.4g that embodiment 1 method prepares, evaluate in fixed-bed reactor: reduction condition: under normal pressure, pure H 2 , the temperature is 350°C, and the space velocity is 1500mL / (h·g cat ), the reduction time is 12h. Reaction condition: H 2 / CO 2 =3.0, the temperature is 320°C, the pressure is 3.0MPa, the space velocity is 2000~20000mL / (h·g cat ), investigated the influence of feed gas space velocity on catalyst performance, test result (see table 3) shows, C 2 = ~C 4 = As the space velocity increases, the selectivity first increases gradually and then begins to decrease. When the space velocity is 8000mL / (h g cat ) reached the maximum; the catalyst at 20000mL / (h·g cat ) airspeed, still maintain high CO 2 conversion (31.8%) and high C 2 = ~C 4 = Selectivity (45.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com