Synthetic method of cefbuperazone intermediate
A technology for cefrazone and a synthesis method, which is applied in the field of synthesis of cefrazone intermediates, can solve the problems of non-compliance with environmental protection requirements, difficulty in separation and purification, and many reaction steps, and achieves low cost, high yield and convenient preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
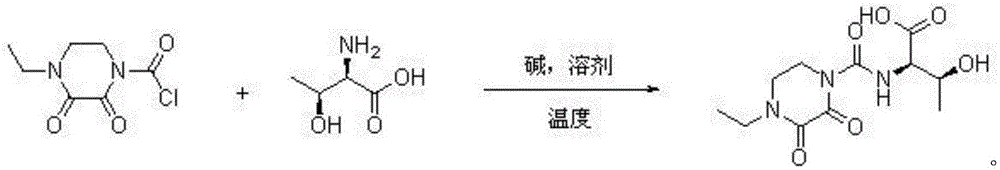
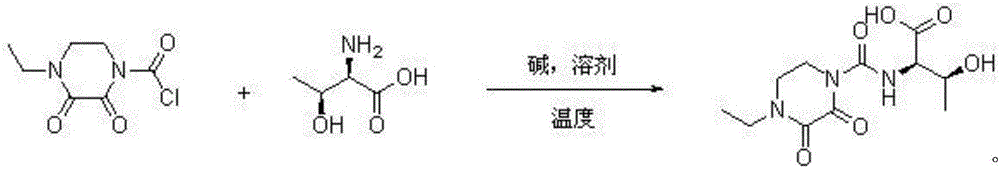
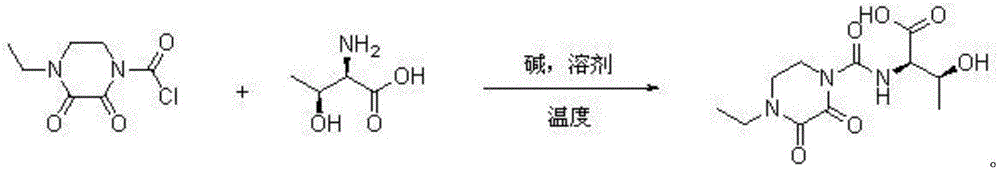
[0026] Embodiment 1: a kind of synthetic method of cefbuperazone intermediate, cefbuperazone intermediate is at normal temperature D-threonine and N-ethyl-2,3-dioxopiperazinyl formyl chloride in alkaline Directly react in the solvent to generate, after the reaction is completed, the layers are separated, the lower layer of the water layer is extracted with dichloromethane to extract small polar impurities, the pH value of the water phase is adjusted to 0 with concentrated hydrochloric acid and concentrated to dryness, dissolved in ethyl acetate as a desalting solvent and After filtration, the filtrate was concentrated to dryness, and then dichloromethane was added at 20°C for slurry purification to obtain the purified cefbuperazone intermediate.
[0027] Wherein, the equivalent ratio of D-threonine and N-ethyl-2,3-dioxopiperazinylcarbonyl chloride is 1:1; the normal temperature is 20°C; the alkali is sodium hydroxide; The solvent is water; the mass volume ratio of the solvent ...
Embodiment 2
[0028] Embodiment 2: a kind of synthetic method of cefbuperazone intermediate, cefbuperazone intermediate is at normal temperature D-threonine and N-ethyl-2,3-dioxopiperazinyl formyl chloride in alkaline It is formed by direct reaction in the solvent. After the reaction is completed, the layers are separated. The lower water layer is extracted with dichloromethane to extract small polar impurities. The pH value of the water phase is adjusted to 6 with concentrated sulfuric acid and concentrated to dryness. After the filtrate was concentrated to dryness, chloroform was added at 80°C for beating and purification to obtain the purified cefbuperazone intermediate.
[0029] Wherein, the equivalent ratio of D-threonine and N-ethyl-2,3-dioxopiperazinylcarbonyl chloride is 1:1.1; the normal temperature is 30°C; the base is potassium hydroxide; The solvent is a mixture of water and dichloromethane, and the volume ratio of dichloromethane to water is 1:10; the mass volume ratio of the s...
Embodiment 3
[0030] Embodiment 3: a kind of synthetic method of cefbuperazone intermediate, cefbuperazone intermediate is at normal temperature D-threonine and N-ethyl-2,3-dioxopiperazinyl formyl chloride in alkaline It is formed by direct reaction in the solvent. After the reaction is completed, the layers are separated. The lower aqueous layer is extracted with dichloromethane to extract small polar impurities. The pH value of the aqueous phase is adjusted to 1 with concentrated phosphoric acid and concentrated to dryness. Dissolve and filter with desalting solvent propionitrile After the filtrate was concentrated to dryness, ethyl acetate was added at 25°C for beating and purification to obtain the purified cefbuperazone intermediate.
[0031] Wherein, the equivalent ratio of D-threonine and N-ethyl-2,3-dioxopiperazinylformyl chloride is 1:1.2; the normal temperature is 22°C; the base is sodium carbonate; The solvent is a mixture of water and dichloromethane, and the volume ratio of dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com