Glycogen phosphorylase inhibitor and preparation method and application thereof
A phosphorylase inhibitor, glycogen phosphorylase technology, applied in the field of drug synthesis, can solve the problem of limited hypoglycemic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
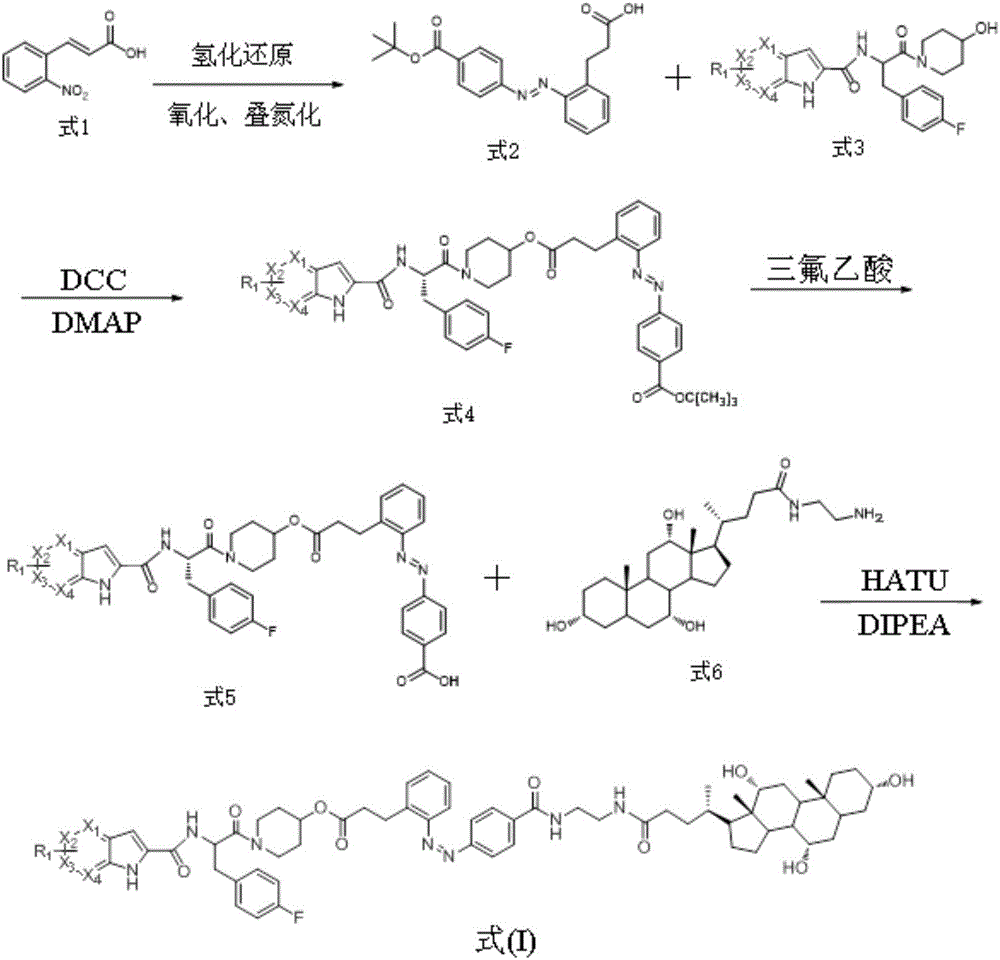
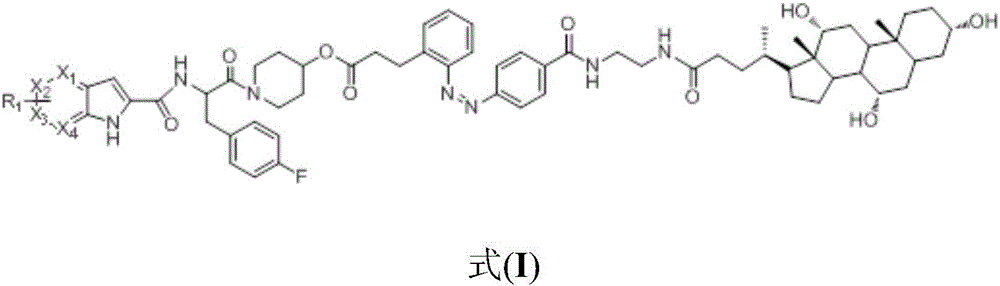
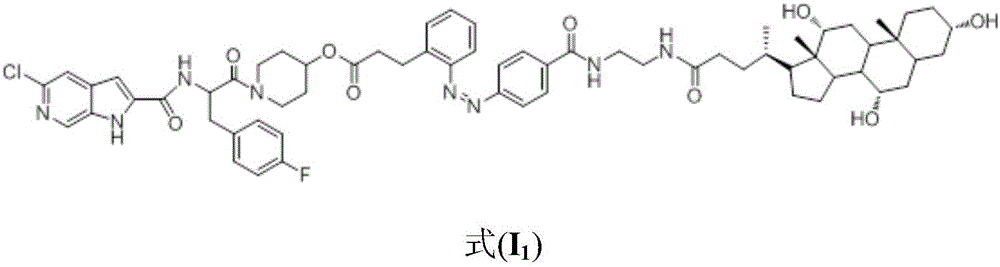
[0038] The preparation of a kind of glycogen phosphorylase inhibitor in this embodiment, the specific preparation steps are as follows:
[0039] (1) Synthesis of (E)-3-{2-[(4-tert-butoxycarbonylphenyl)azido]phenyl}propionic acid:
[0040] (E)-3-(nitrophenyl)acrylic acid (10.0g, 52.0mmol) was dissolved in water (500mL), NaOH (4.1g, 39mmol) and 10%Pd / C (0.5g) were added, hydrogenated at room temperature and atmospheric pressure overnight. The reactant was filtered to remove Pd / C, and the solvent was evaporated under reduced pressure to obtain a white powdery solid crude product (7.5 g, 87%). This crude product was dissolved in a mixed solvent of water (200 mL) and dichloromethane (300 mL), and potassium hydrogen persulfate (Oxone, 55.3 g, 90.0 mmol) was added, and stirred at room temperature for 2 h. The organic phase of dichloromethane was collected by extraction, the aqueous phase was extracted with dichloromethane, the organic phases were combined, dried over anhydrous sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com