Method for determining zebrafish M receptor level based on bio-luminescence method
A bioluminescence and zebrafish technology, applied in the field of physiological ecology, can solve the problems of human hazards for experimenters, cumbersome technical steps, expensive reagents, etc., and achieve the effects of low cost, simple and fast operation, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Break the zebrafish tissue, centrifuge at low temperature, take the supernatant, and place equal amounts of the supernatant in three incubation tubes A, B, and C;
[0031] (2) Convert GDP and GMP in the supernatant obtained in step (1) into GTP; more specifically, first add 50 μL of supernatant to each incubation tube, and then add pH7.5 zwitterions to each tube Buffer, MgCl 2 After that, tube A will not add any substances, tube B will add a certain amount of phosphoenolpyruvate and pyruvate kinase, tube C will add a certain amount of ATP and guanylate kinase, and the reaction mixture in each incubation tube will be uniform. Adjust to 250 μL with ultrapure water; incubate the reaction mixture and then boil the water bath to terminate the reaction, then place the incubation tube containing the reaction mixture in ice to cool, the incubation temperature is 30°C, and the time is 30min; the cooling The time is 10 minutes;
[0032] (3) Destroy the ATP and UTP in each r...
experiment example 1
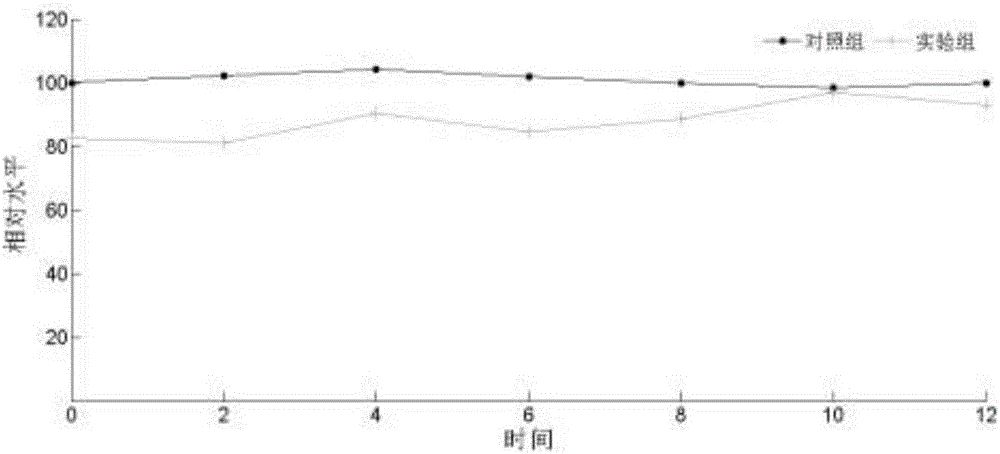
[0040] 1. The experiment was divided into experimental group and control group. Six zebrafish were placed in each group, and each zebrafish was used as a sample. Samples were taken at 2h, 4h, 6h, 8h, 10h, and 12h. The experimental group was exposed to 0.01mol / L atropine (an anticholinergic drug that blocks M receptors) for 12 hours, and the control group did not add any substance.
[0041] 2. Take out the sample and add it to pre-cooled PBS, fully homogenize it in an ice bath, then centrifuge at 12,000 rpm at 4°C for 20 minutes, take the supernatant, and process it through the following steps respectively.
[0042] Three separate incubation tubes, A, B, and C, were prepared for the experimental group and the control group respectively, and were processed by the following steps:
[0043] (1) Convert GDP and GMP in the obtained zebrafish supernatant to GTP; more specifically, first add 50 μL of supernatant to each incubation tube, and then add pH7.5 zwitterions to each tube Buf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com