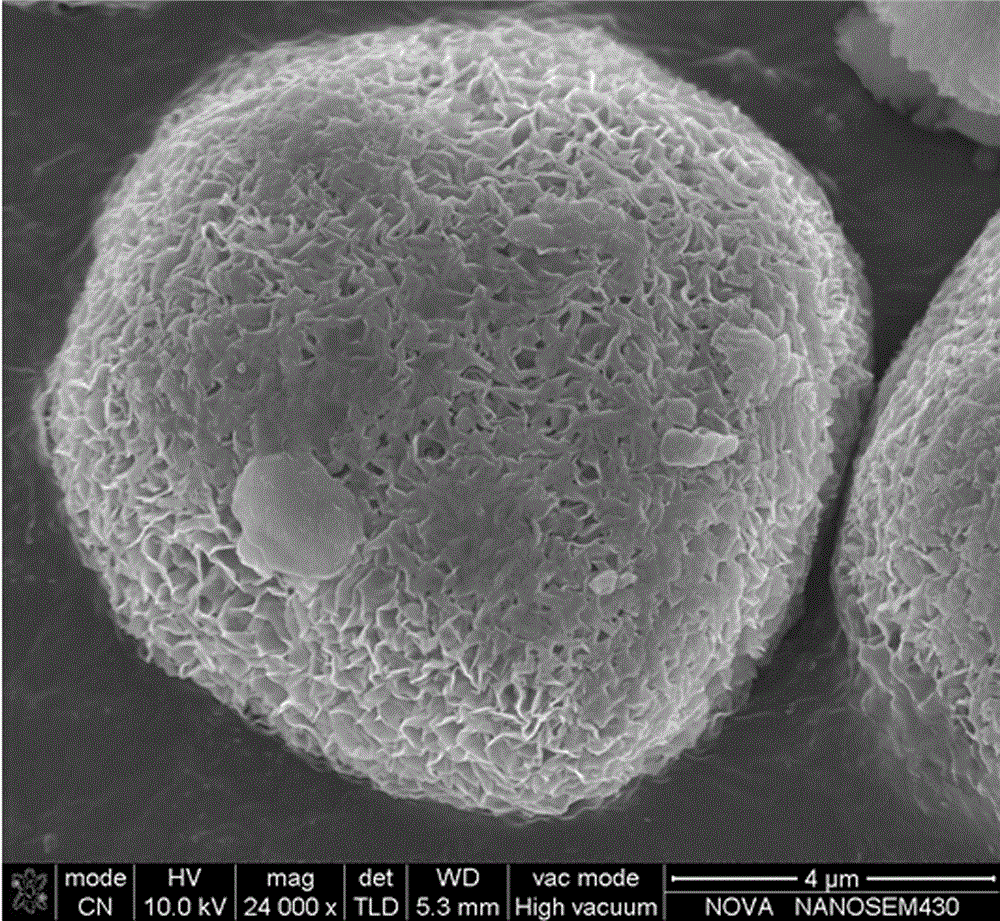
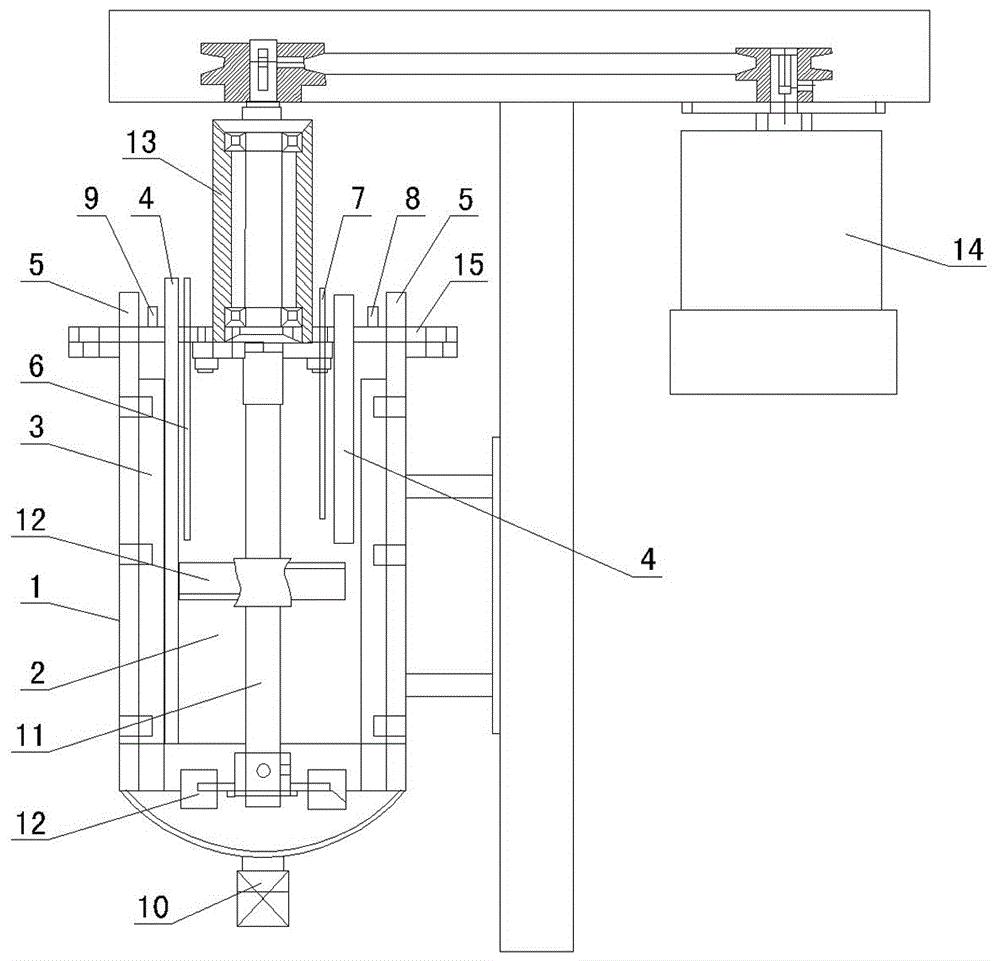
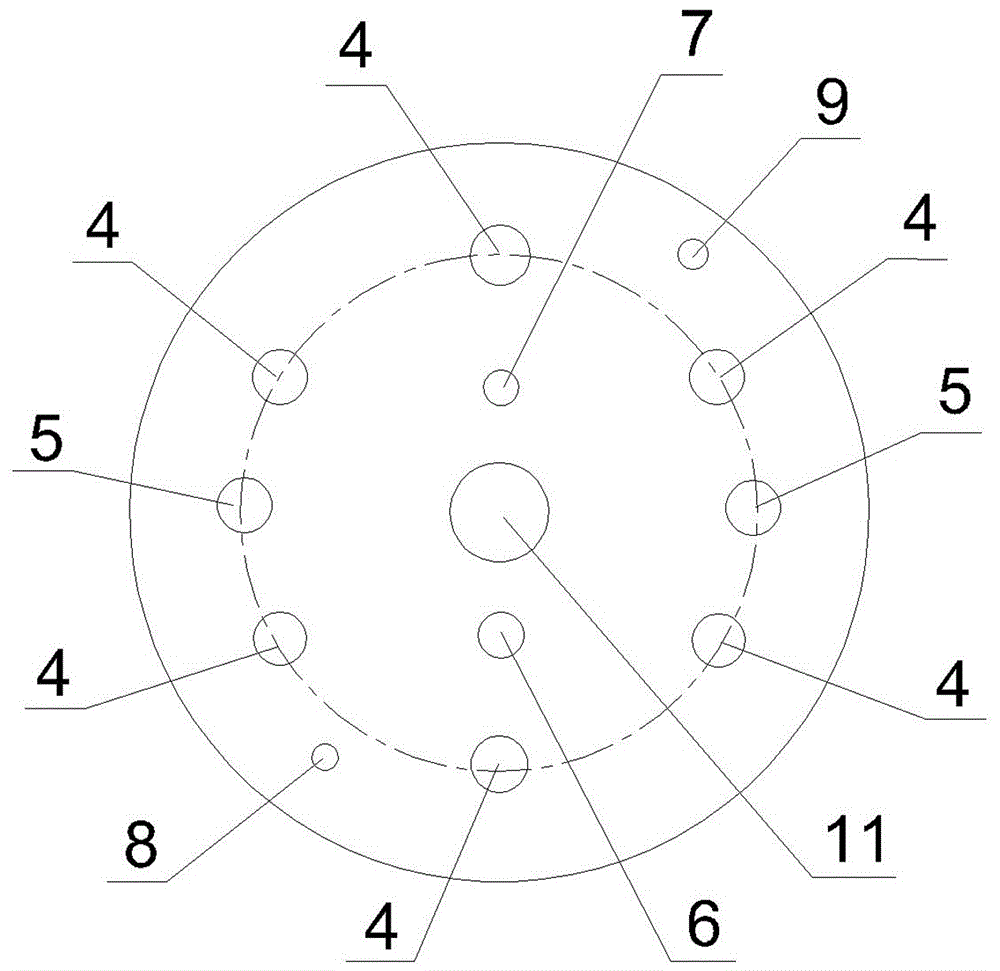
Preparation method and device of nickel-cobalt-aluminum anode material precursor
A technology for positive electrode materials and preparation devices, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of difficult to form spherical large particle precipitation, fast aluminum ion precipitation, and unsatisfactory NCA material performance indicators, and achieve fast diffusion speed. , The process is simple and easy to control, and the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the nickel-cobalt-aluminum cathode material precursor of the present invention comprises the following steps:
[0031] 1) Prepare nickel-cobalt-aluminum mixed salt solution containing complexing agent and strong alkali solution containing ammonia water; wherein, the preparation method of nickel-cobalt-aluminum mixed salt solution containing complexing agent is to dissolve soluble aluminum salt and complexing agent in In deionized water, after the aluminum ion reacts with the complexing agent to form an aluminum complex (dissolve and stir for 5-15 minutes), then add soluble nickel salt and soluble cobalt salt; and the concentration of metal ions in the nickel-cobalt-aluminum mixed salt solution is 0.5 ~3.5mol / L, and the total molar ratio of nickel, cobalt, and aluminum ions can be formulated according to the molar ratio of nickel, cobalt, and aluminum in the precursor of the nickel-cobalt-aluminum cathode material to be prepared; wherein, soluble...
Embodiment 1
[0037] Dissolve 80g of a complexing agent formed by ammonia water and sodium salicylate in 3L of deionized water, and add 152g of Al 2 (SO 4 ) 3 18H 2 O, after dissolving, continue stirring for 10min and then add 1912g NiSO 4 ·6H 2 O and 384g CoSO 4 ·7H 2 0, stirring and dissolving to obtain the nickel-cobalt-aluminum mixed salt solution containing complexing agent, particularly emphasized that the mol ratio of nickel, cobalt, aluminum ions and the mol ratio of nickel-cobalt-aluminum in the nickel-cobalt lithium aluminate material that needs to prepare are Consistent, that is, both are 0.8︰0.15︰0.05, to ensure that the proportion of nickel-cobalt-aluminum in the precursor formed is consistent with the required proportion of nickel-cobalt-lithium aluminate material at any time during the reaction process, and the material The composition is uniform at any time; prepare 9L of sodium hydroxide solution with a concentration of 6M, add ammonia water and mix evenly to obtain a...
Embodiment 2
[0040] Add 10g of ethylene glycol as a complexing agent into 3L of deionized water, and after it dissolves, add 101g of Al 2 (SO 4 ) 3 18H 2 O, continue to stir for 15min after dissolving, after adding 227g C 4 h 6 o 4 Co 4H 2 O and 1195g C 4 h 6 o 4 Ni·4H 2 0 is stirred until dissolving to obtain the nickel-cobalt-aluminum mixed salt solution that contains complexing agent; Configuration 9L concentration is the sodium hydroxide solution of 5M, adds ammoniacal liquor and mixes and obtains the strong alkali solution that ammonia concentration is 0.8M;
[0041] Add 3L of bottom liquid into the 20L reaction kettle, and after heating to 60°C, the stirring speed is controlled at 200-250r / min. The flow rate is 0.35L / h, adjust the flow rate of the strong alkali solution to control the pH value of the solution in the reactor between 10.5 and 11.5; after the nickel-cobalt-aluminum mixed salt solution is added, stop adding the strong alkali solution, and discharge the slurry f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com