Novel (r)-hydroxynitrile lyase
A lyase and hydroxynitrile technology, which is applied in the field of preparation of the hydroxynitrile lyase, can solve the problems that the expression amount and biochemical activity cannot be directly applied, and the biochemical activity of the target protein of the transformant cannot be easily predicted in advance, and the like, achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077]
[0078] The (R)-hydroxynitrile lyase of the present invention can be produced by culturing the above-mentioned transformant and refining it from the obtained culture.
[0079] The "culture" in the present invention refers to any one of culture supernatant, cultured cells, cultured cells, or crushed products of cells or cells. The culture obtained by culturing the transformant of the present invention is included in the scope of the present invention.
[0080] The culture of the transformant of the present invention can be carried out according to the usual method used in host culture. The target (R)-hydroxynitrile lyase is accumulated in the above culture.
[0081] The culture medium for cultivating the transformant of the present invention contains carbon sources, nitrogen sources, inorganic salts, etc. that can be assimilated by the host, and it is sufficient that the culture medium for effectively cultivating the transformant can be used. A sort of. Examples of...
Embodiment 1
[0093] Embodiment 1: the acquisition of natural type (R)-hydroxynitrile lyase
[0094] (1) Purification of (R)-hydroxynitrile lyase
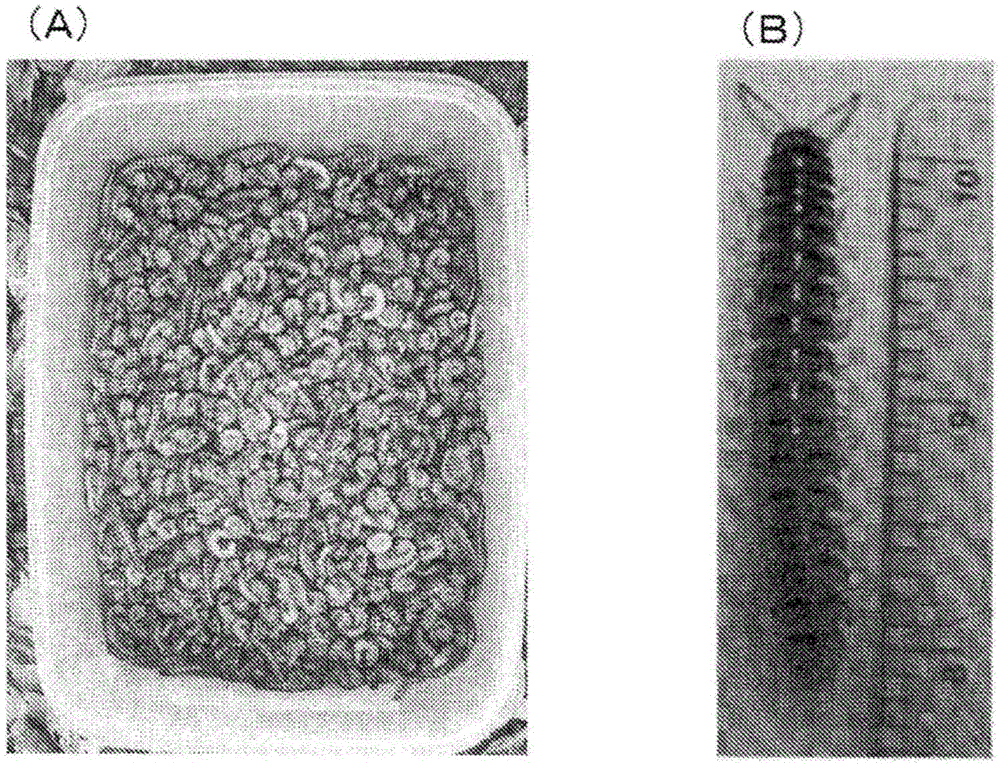
[0095]It was caught in November 2010, November 2011 and August 2012 when the calamity of Chamberlinius hualienensis was confirmed in Kagoshima Prefecture. The captured millipedes were cooled in a container with dry ice, and stored at -80°C until use. figure 1 For the photo of the living millet.
[0096] The cryopreserved above-mentioned millipedes (2 kg in total) were ground in liquid nitrogen using a mortar and pestle. The obtained microparticles were suspended by stirring in 20 mM potassium phosphate buffer (pH 7.0) for 3 hours or more under ice-cooling. The resulting suspension was carefully filtered through overlapping layers of cotton gauze to remove solids. The obtained solution was treated with protamine sulfate (manufactured by NACALAI TESQUE) obtained from salmon semen for 30 minutes, and then centrifuged at 4° C. and 28,500×g for 3...
Embodiment 2
[0113] Embodiment 2: Structural analysis of natural type (R)-hydroxynitrile lyase
[0114] (1) Analysis of molecular weight and quaternary structure
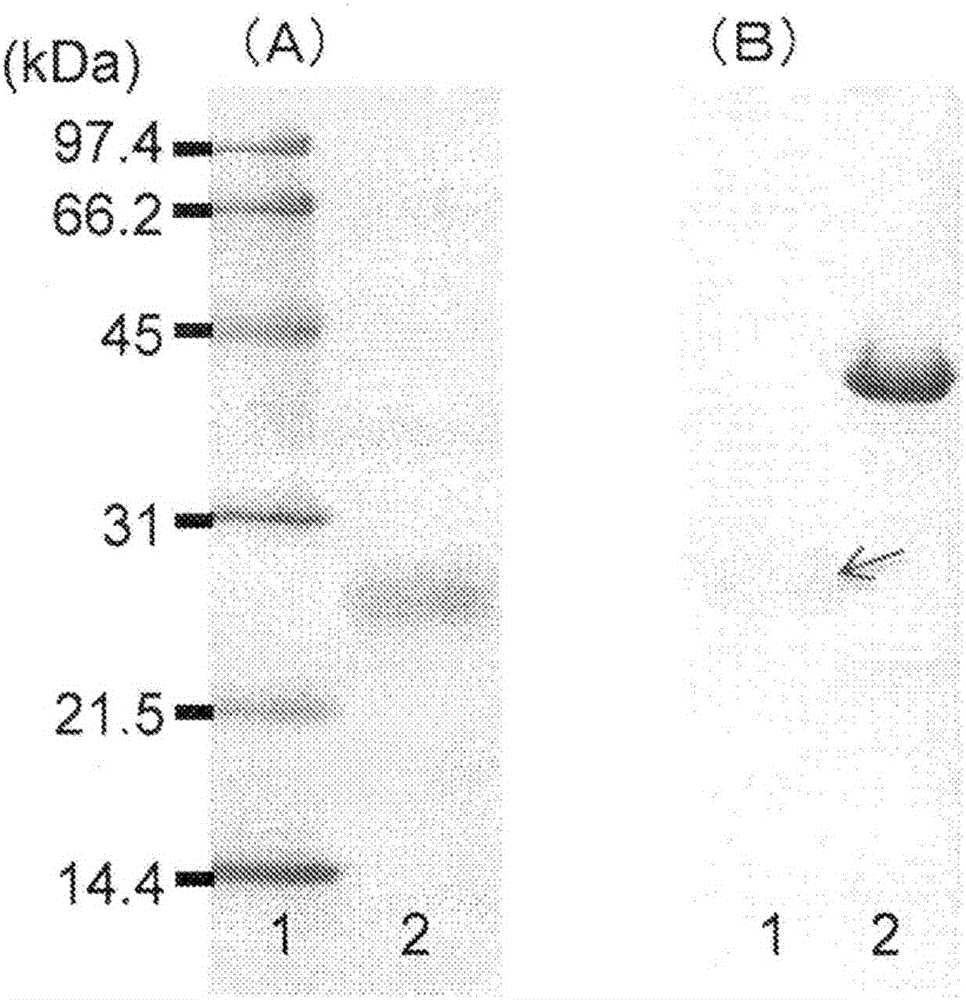
[0115] The size of the monomeric subunit of R-ChHNL purified in Example 1 above was analyzed by SDS-PAGE using a molecular weight marker (manufactured by Bio-Rad). The molecular weight and quaternary structure were determined by gel filtration column chromatography using Superdex 10 / 300GL manufactured by GE Healthcare. For details, refer to Dadashipour et al., Journal of Biotechnology, 153, pp. 100-110 (2011) (hereinafter, this document will be abbreviated as "Dadashipour et al. (2011)"). The results of SDS-PAGE are as follows figure 2 (A) shown. figure 2 In (A), 1 is the lane of molecular weight markers of 97.4 kDA, 66.2 kDa, 45 KDa, 31 kDa, 21.5 kDa and 14.4 kDa from the top, and 2 is the lane of purified R-ChHNL.
[0116] From the results of SDS-PAGE and gel filtration, it can be known that the molecular weight of R-ChH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com