New preparation method of silodosin compound
A silodosin and compound technology, which is applied in the new preparation field of silodosin, can solve the problems of being unsuitable for industrial scale production, having many impurities or side reactions, and low product purity, avoiding functional group protection and deprotection, The effect of reducing the purification and impurity removal process and simplifying the process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
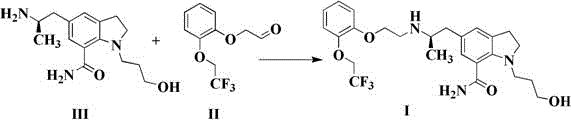
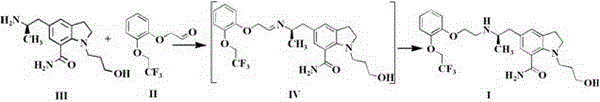
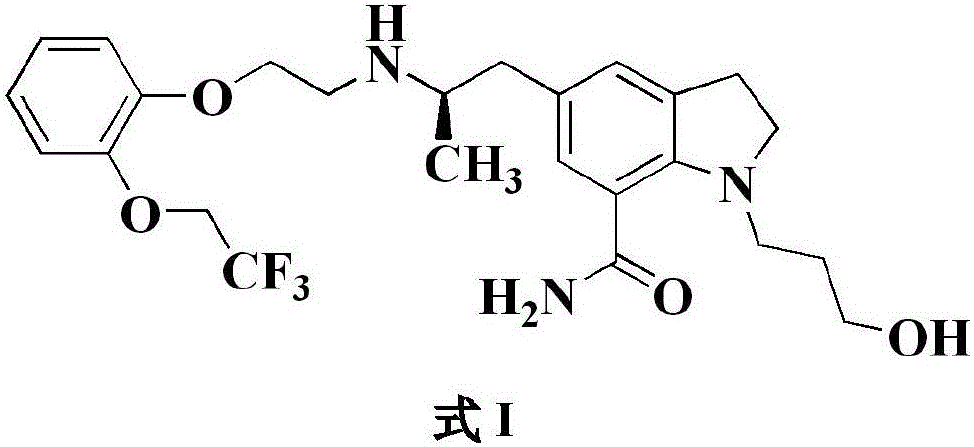
[0031] Add 10.0 g (42.7mmol) of compound II, 13.0 g (47.0mmol, 1.1eq) of compound III to 200ml of methanol, add 130 mg (2.1mmol, 0.05eq) of acetic acid, and then add 54.0g (256mmol, 6.0eq) of three Sodium acetoxyborohydride, stir and react at 20~30℃ for 0.5~1h; adjust the pH of the reaction solution to 7~8 with 1mol / L sodium hydroxide solution, add 1L ethyl acetate to extract the layers, and concentrate the organic phase to 70ml by subtracting evaporation , The temperature was lowered to 0-10 ℃ and crystallized to obtain 18.1 g of xerodoxine, the yield was 85.6%, the HPLC purity was 99.85%, and the impurity P1 content was 0.02%.
Embodiment 2
[0033] 1.0 g (4.27mmol) of compound II and 2.4 g (8.54mmol, 2.0eq) of compound III were added to 20ml of methanol, 0.74g (4.27 mmol, 1.0eq) of p-toluenesulfonic acid was added, and 1.1g (17.1 mmol, 4.0eq) Sodium cyanoborohydride, stirred at 20~30℃ for 0.5~1h; adjust the pH of the reaction solution to 7~8 with 1mol / L sodium hydroxide solution, add 100ml ethyl acetate to extract the layers, and concentrate the organic phase by distillation To 10ml, the reduced distillate was cooled to 0~10°C and crystallized to obtain 1.4g of xerodoxine, the yield was 66.2%, the HPLC purity was 99.89%, and the content of impurity P1 was 0.01%.
Embodiment 3
[0035] Add 1.0 g (4.27mmol) of compound II, 1.3 g (4.70 mmol, 1.1eq) of compound III to 20ml of ethanol, add 25mg (0.42mmol, 0.1eq) of acetic acid, and then add 0.65g (17.1 mmol, 4.0eq) of boron Sodium hydride, stirring at 20~30℃ for 0.5~1h; adjust the pH of the reaction solution to 7~8 with 1mol / L sodium hydroxide solution, add 100ml ethyl acetate to extract the layers, the organic phase is subtracted and concentrated to 10ml, and the reduced distillate The temperature was lowered to 0-10°C to crystallize to obtain 1.5g of xerodoxine, the yield was 70.9%, the HPLC purity was 99.86%, and the impurity P1 content was 0.02%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com