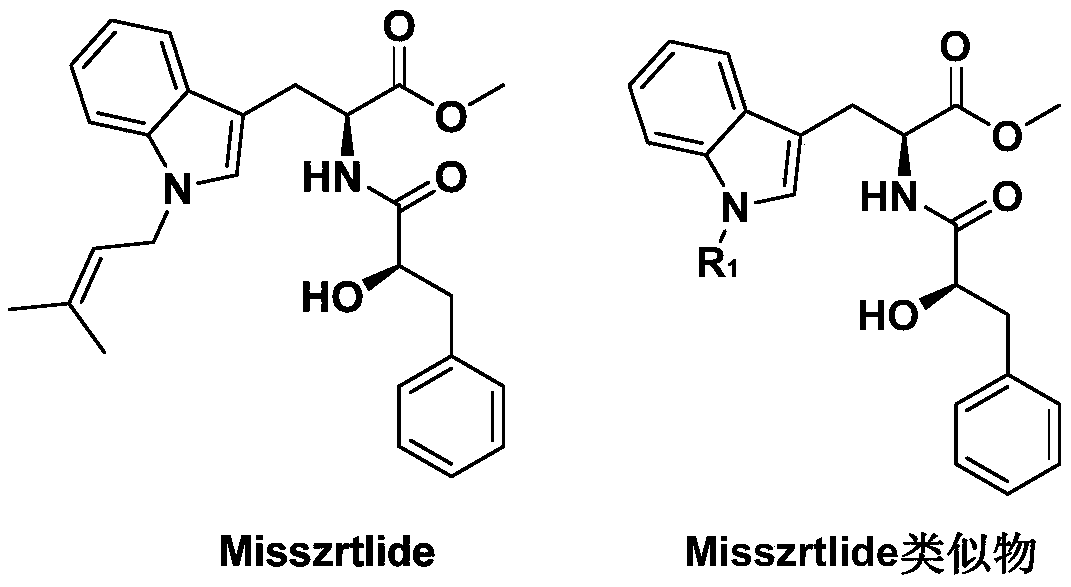
A kind of synthetic method of active alkaloid misszrtlide
A technology of active alkaloid and synthesis method, which is applied in the field of synthesis of active indole alkaloid Misszrtlide, can solve the problems of inability to meet actual clinical research and industrial application requirements, less difficulty in collecting alkaloids, etc., so as to reduce synthesis time and Purification process, improved yield and control of by-products, the effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
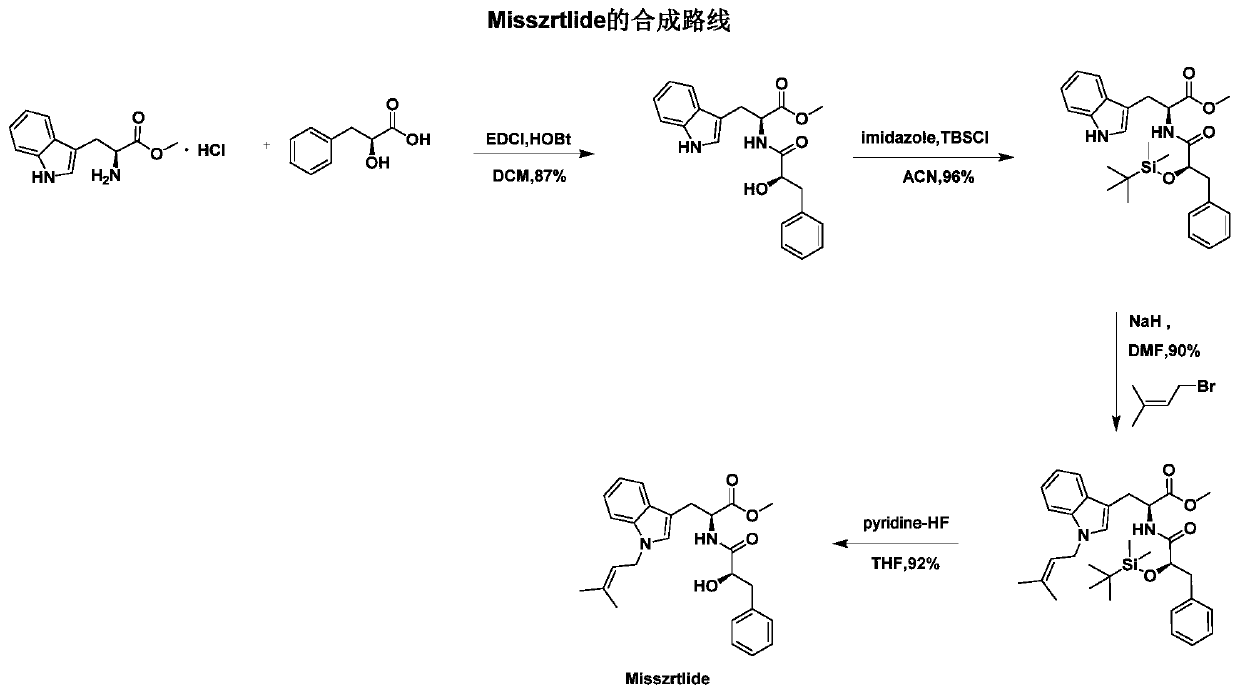
[0045] Embodiment 1: the synthesis of active alkaloid Misszrtlide
[0046] (1) Coupling of amides
[0047] In a 250 mL round bottom flask, 4.0 g of L-phenyllactic acid (L-(-)-3-Phenyllactic acid, 24 mmol) was dissolved in 100 mL of dry dichloromethane (DCM), and then 3.8 g of 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 20mmol) was reacted for 5min, then 2.7g of 1-hydroxybenzotriazole (HOBt, 20mmol) was added and stirred for 60min Afterwards, 5.1 g of L-tryptophan methyl ester hydrochloride (H-Trp-OMe HCl, 20 mmol) was added thereto, nitrogen gas was introduced, and the reaction was carried out at room temperature (25-29° C.) for 30 h, and thin-layer chromatography ( TLC) follow the reaction, after the reaction is complete, spin dry under reduced pressure, extract three times with dichloromethane (DCM) and water, lyophilize the organic phase, and then separate through column chromatography to obtain N α -((R)-2-Hydroxy-phenylpropionyl)-L-tryptophan met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com