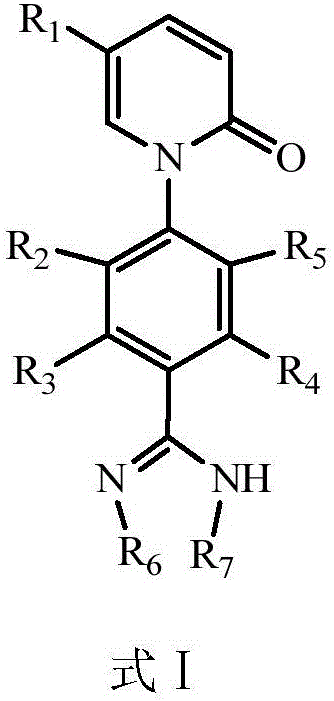
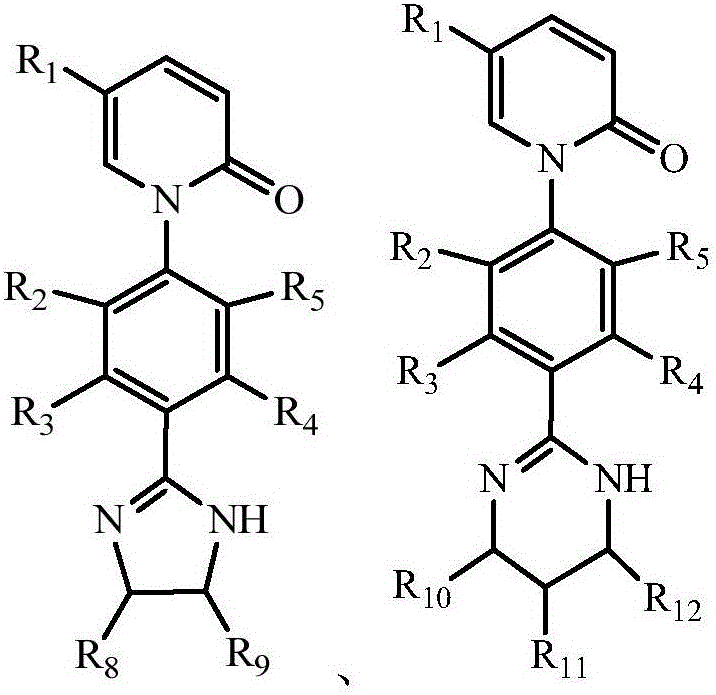
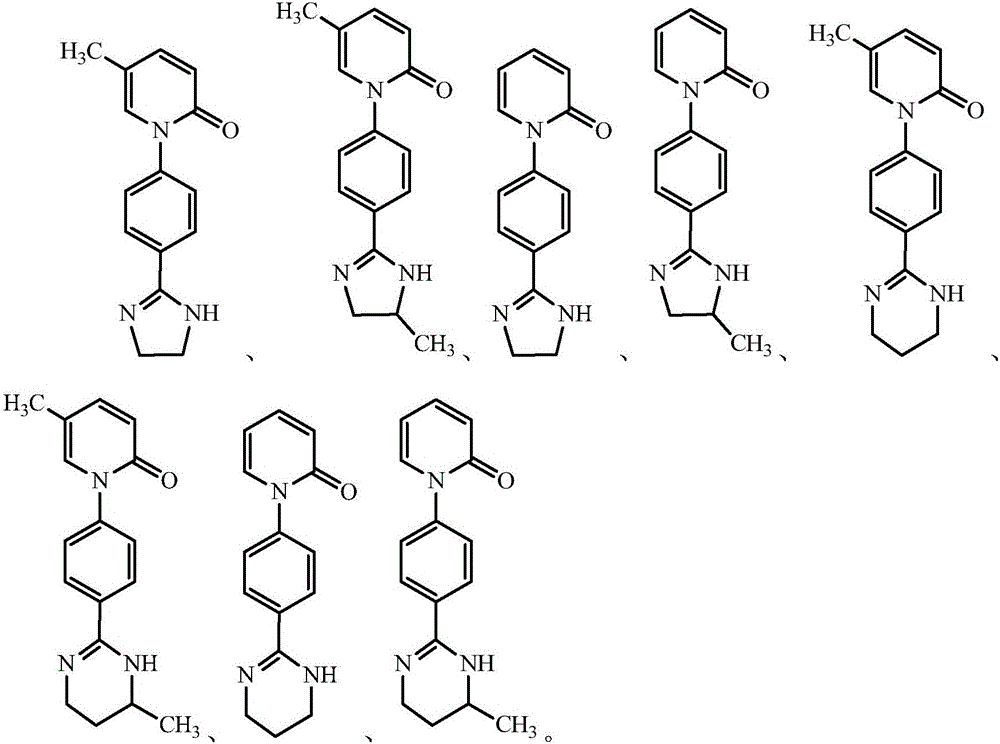
Pirfenidone derivative and preparation method thereof
A compound and hydrate technology, applied in the field of pirfenidone derivatives and its preparation, can solve the problems of poor anti-fibrosis activity and achieve good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, the synthesis of compound 5b of the present invention
[0050] synthetic route:
[0051]
[0052] 1. Synthesis of compound 3 (5-methyl-2-(1H)-pyridone)
[0053] First add 3.40mL of 50% sulfuric acid (v / v) to a 25mL reaction flask, then add 1.00g (10mmol) of 2-amino-5-picoline (compound 1), cool to below 10°C in an ice-salt bath, and stir for several After 10 minutes, the reaction solution turned milky white; then slowly added dropwise 1.72g (25mmol) NaNO 2 with 3mL H 2 The mixed solution composed of O has brown-yellow gas with pungent odor during the dropwise addition process. After the addition, the reaction solution turns light yellow. Use 10% dilute sulfuric acid to adjust the pH to 7-8, reflux and stir for about 20 minutes, and spin Most of the water was removed, an appropriate amount of 300 mesh silica gel was added thereto, spin-dried, poured into a glass sand core funnel, rinsed with ethyl acetate and suction-filtered, and the filtrate was spi...
Embodiment 2
[0062] Embodiment 2, the synthesis of compound 5c of the present invention
[0063] According to the method similar to Example 1, in step 3, ethylenediamine was used as a raw material to prepare compound 5c, and the single-step yield of step 3 was 69%.
[0064]
[0065] Compound 5c: 1-(4-(4,5-dihydro-1H-imidazol-2-yl)phenyl)-5-methylpyridin-2(1H)-one, yellow solid, m.p.252-254°C;
[0066] 1 H NMR (400MHz, DMSO) δ8.01 (d, J = 8.5Hz, 2H), 7.68 (d, J = 8.5Hz, 2H), 7.55–7.31 (m, 2H), 6.45 (d, J = 9.3Hz ,1H),4.02–3.25(m,4H),2.05(s,3H),1.86(dd,J=12.6,9.6Hz,1H);
[0067] 13 C NMR (101MHz, DMSO) δ164.04, 160.29, 144.87, 143.66, 135.37, 129.01, 127.50, 125.22, 123.22, 120.32, 114.84, 69.80, 45.61, 16.42;
[0068] HRMS (ESI) calcd for C 15 h 15 N 3 O[M+H] + 254.1294, found 254.1289.
Embodiment 3
[0069] Embodiment 3, the synthesis of compound 5d of the present invention
[0070] According to the method similar to Example 1, using 1,3-propylenediamine as raw material in step 3, compound 5d was prepared, and the single-step yield of step 3 was 54%.
[0071]
[0072] Compound 5d: 1-(4-(1,4,5,6-tetrahydropyrimidin-2-yl)phenyl)-5-methylpyridin-2(1H)-one, yellow solid, m.p.265-267℃ ;
[0073] 1 H NMR (400MHz, DMSO) δ7.83 (d, J = 8.5Hz, 2H), 7.68 (d, J = 8.5Hz, 2H), 7.51–7.40 (m, 2H), 6.45 (d, J = 9.3Hz ,1H),3.51(m,6H),2.93(dd,J=14.7,7.4Hz,1H),2.05(d,J=13.5Hz,3H);
[0074] 13 C NMR (101MHz, DMSO) δ160.21, 158.66, 144.51, 143.61, 135.29, 128.51, 127.95, 127.39, 120.27, 114.62, 45.76, 17.60, 16.37, 11.06;
[0075] HRMS (ESI) calcd for C 16 h 17 N 3 O[M+H] + 268.1451, found 268.1449.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com