Olaparib pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, sugar-coated pills, etc., can solve problems such as poor stability, drug safety of patients affecting drug shelf life, stomach discomfort, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
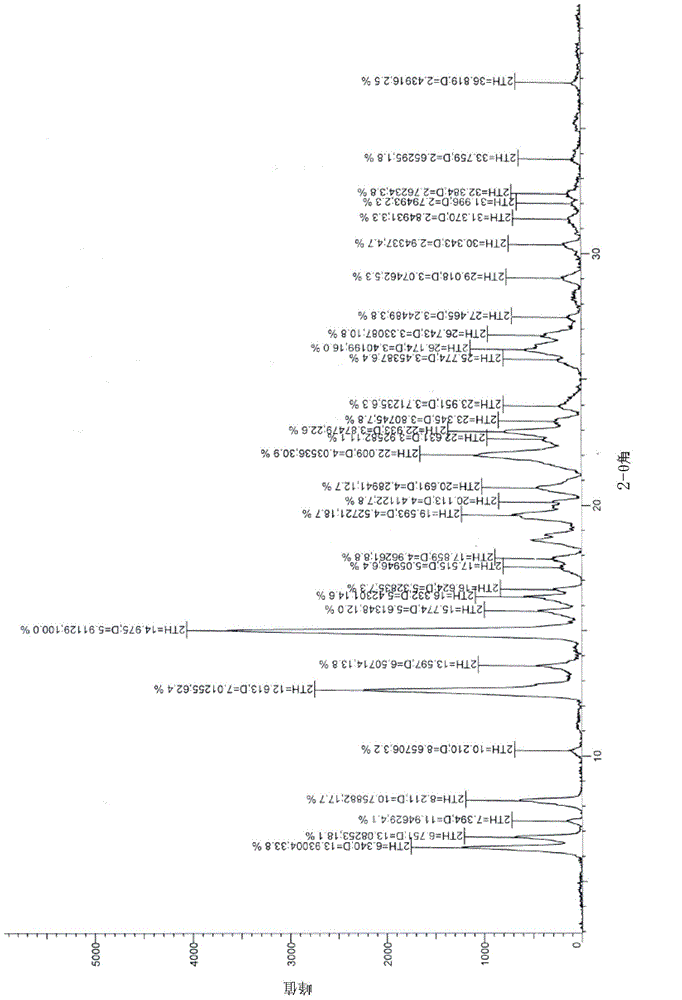
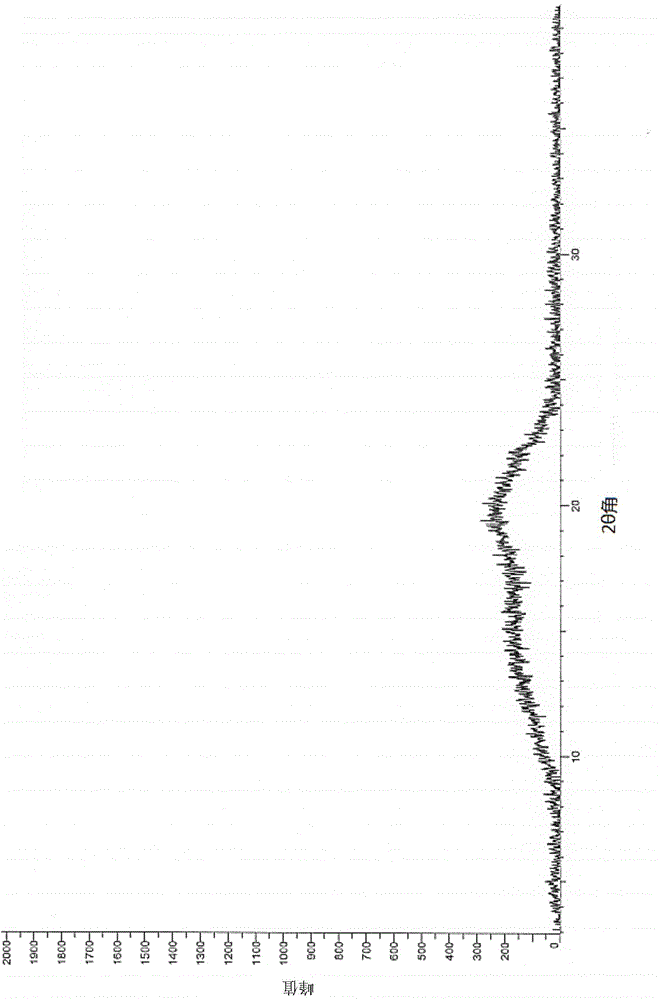
Image
Examples
Embodiment 1
[0049] Table 1: Prescription Composition of Olaparib Tablets
[0050]
[0051] After olaparib, povidone 12PF and talcum powder are mixed evenly according to the prescription ratio, they are added into a drug hot-melt extruder to prepare a solid dispersion, which is cooled and crushed into suitable granules. Then the above-mentioned granules are evenly mixed with mannitol and remaining talcum powder, and magnesium stearate is added for total mixing. Then the mixed granules are pressed into tablets, and the hardness of the tablets is controlled to be ≥6kg / cm 2 . The tablet core is coated, and the weight of the coating is increased by 3%, and finally the finished product of the olaparib tablet is obtained after being packaged in aluminum-plastic blisters.
Embodiment 2
[0053] Table 2: Prescription Composition of Olaparib Tablets
[0054]
[0055] Using the above-mentioned prescription process, the tablet core is coated, and the weight of the coating is increased by 2%, and finally the finished product of olaparib tablet is obtained after being packaged in aluminum-plastic blisters.
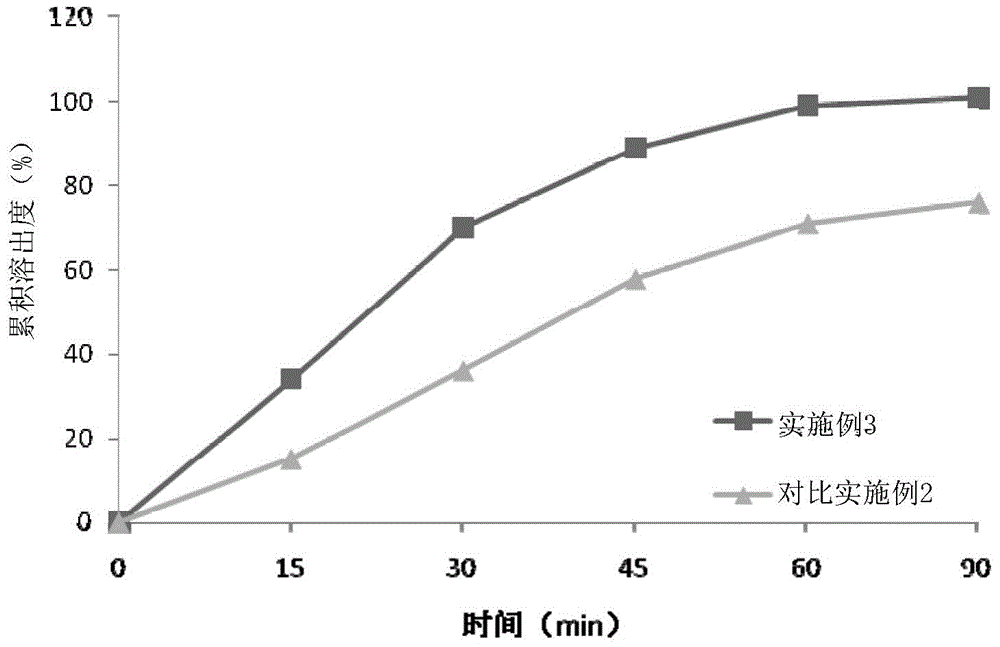
Embodiment 3
[0057] Table 3: Prescription Composition of Olaparib Tablets
[0058]
[0059] Using the above-mentioned prescription process, the tablet core is coated, and the weight of the coating is increased by 3%, and finally the finished product of the olaparib tablet is obtained after being packed in aluminum-plastic blisters.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com