Porous carbon loaded double-crystalline phase cobalt-based fischer-tropsch symthesis catalyst and preparation method and application thereof
A cobalt-based, porous carbon technology, applied in chemical instruments and methods, preparation of liquid hydrocarbon mixtures, catalysts for physical/chemical processes, etc. The effect of low temperature and reduced design requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
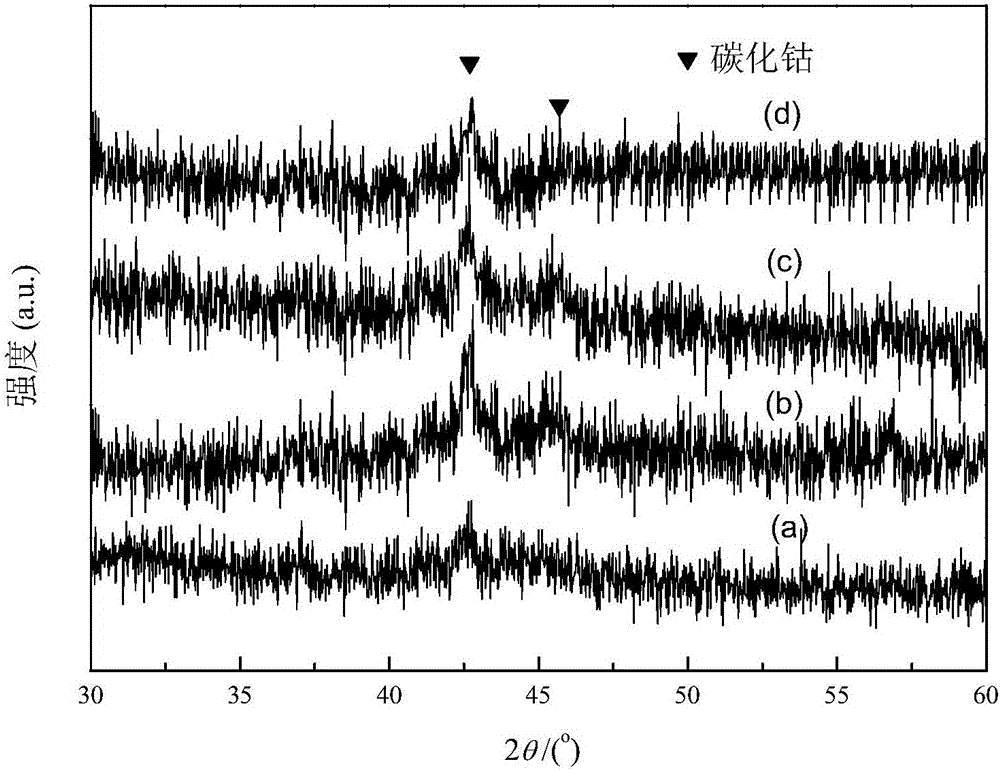
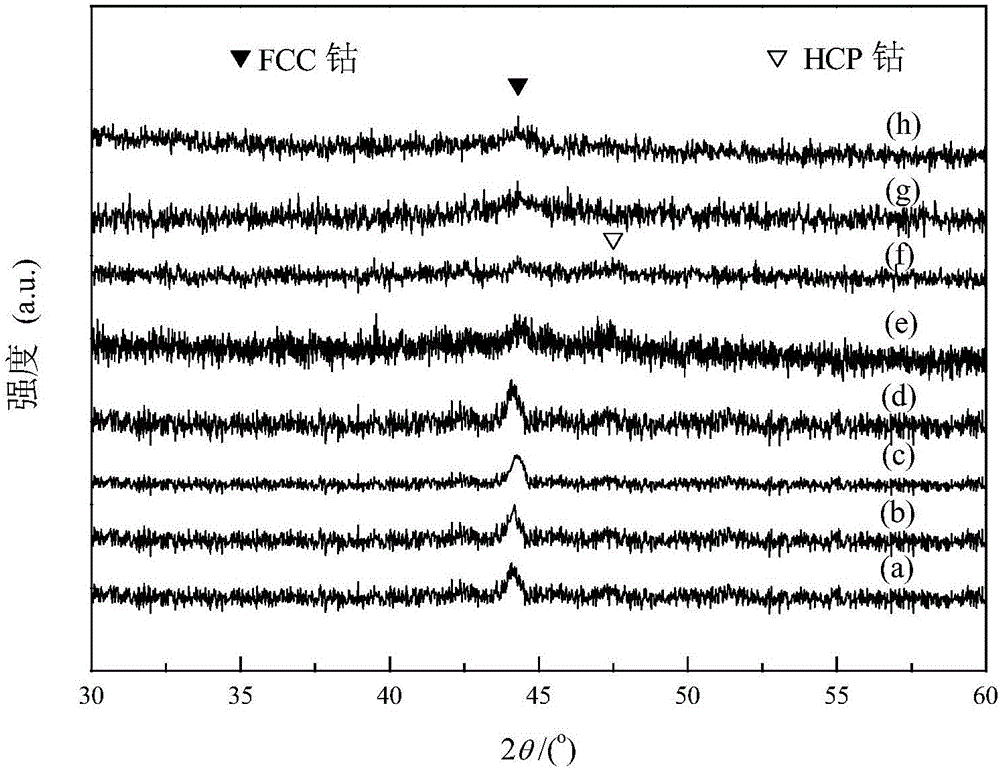
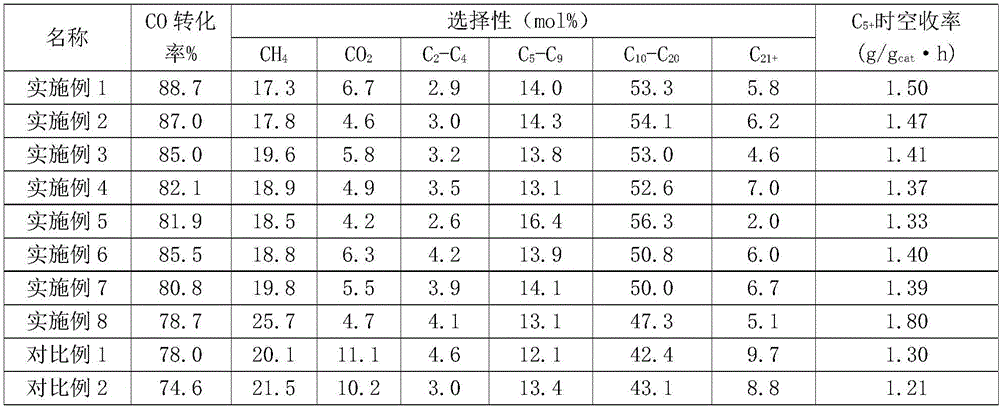
Image
Examples
Embodiment 1
[0035] 0.8g cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O) and 0.46g terephthalic acid (H 2 BDC) is placed in a 100mL Shrek tube, add a mixed solution formed by 20mL DMF and 5mL absolute ethanol, and then use N 2 The air in the tube was replaced with gas and sealed well, heated to 110°C for 15h, then filtered while hot, washed three times with 100mL DMF, and finally dried in air at 100°C for 6h to obtain Co-MOF-71 metal organic framework material.
[0036] 1.48g Co-MOF-71 was placed in a fixed-bed stainless steel reactor, and in a He atmosphere, the temperature was raised to 500°C at a heating rate of 5°C / min for in-situ pyrolysis for 4 hours to obtain a porous carbon-supported cobalt metal intermediate material. Then cool down to room temperature, cut into pure CO gas, the flow rate is 30mL / min, increase the pressure to 2MPa, raise the temperature to 250°C at a heating rate of 5°C / min, keep it for 120h, then cool down to room temperature, use a flow rate of 10mL / min 1 %...
Embodiment 2
[0040] 0.8g cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O) and 0.46g terephthalic acid (H 2 BDC) was placed in a 100mL Shrek tube, and a mixed solution formed by adding 20mL DMF and 5mL absolute ethanol was then used to 2 Gas replaced the air in the tube and sealed it well, raised the temperature to 100°C for 10h, then filtered it while it was hot, washed it three times with 100mL DMF, and finally dried it in air at 80°C for 12h to obtain Co-MOF-71 metal-organic framework material.
[0041] 1.48g Co-MOF-71 was placed in a fixed bed reactor, and in a He atmosphere, the temperature was raised to 500°C at a heating rate of 1°C / min for in-situ pyrolysis for 8h to obtain a porous carbon-supported cobalt metal intermediate material, and then Cool down to room temperature, cut into pure CO gas, the flow rate is 30mL / min, increase the pressure to 2MPa, raise the temperature to 250°C at a heating rate of 5°C / min, keep it for 140h, then cool down to room temperature, use a flow rat...
Embodiment 3
[0045] Using the Co-MOF-71 synthesized in Example 1 as a sacrificial template, place 1.48g of Co-MOF-71 in a fixed-bed reactor, and raise the temperature to 550°C at a heating rate of 1°C / min in a He atmosphere In situ pyrolysis for 8 hours to obtain a porous carbon-supported cobalt metal intermediate material, then cooled to room temperature, cut into pure CO gas, the flow rate was 30mL / min, the pressure was increased to 3MPa, and the temperature was raised to 250°C at a heating rate of 5°C / min. Keep for 120h to obtain a porous carbon-supported cobalt carbide intermediate material;
[0046] Take 0.1g porous carbon-supported cobalt carbide intermediate material in H 2 In the atmosphere, the temperature was raised to 450°C at a heating rate of 5°C / min, and kept for 4 hours to obtain a porous carbon-supported bicrystalline cobalt metal catalyst. The mass percentage of cobalt in this material was 30.1% by atomic absorption spectroscopy (AAS). The reaction conditions of the catal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com