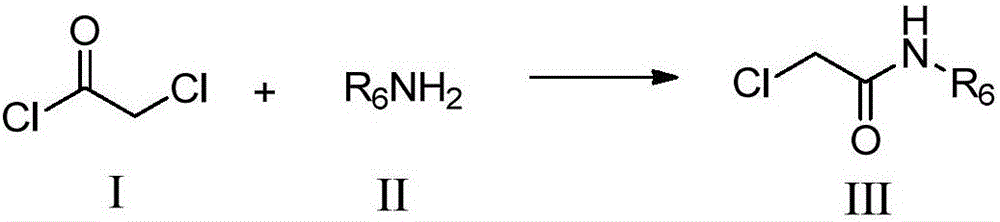Quinazoline dione derivatives and preparation method and application thereof
A kind of technology of quinazoline dione and derivatives, applied in the field of medicine, can solve problems such as unsatisfactory drug effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
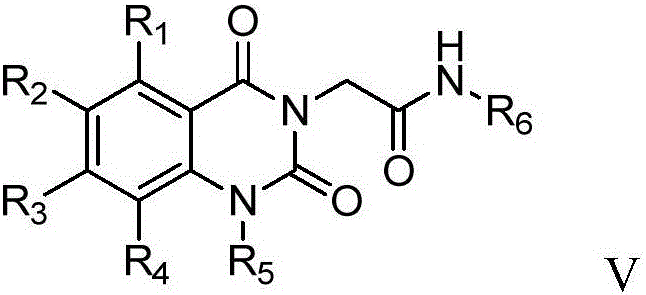
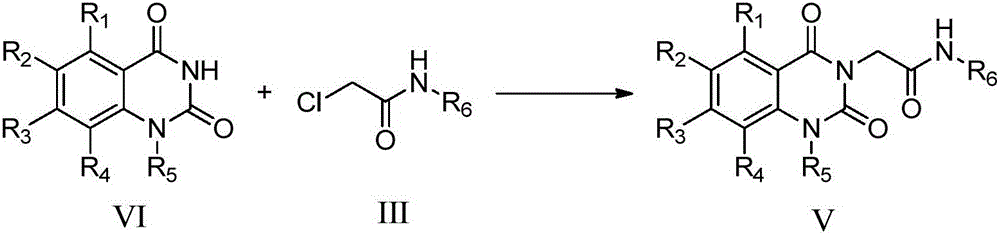
[0101] Example 1: N-tert-butyl-2-(1-cyclopropylmethyl-5-methyl-2,4-dione-1,2-dihydroquinazolin-3(4H)-yl)ethyl Amide (compound 1)
[0102] a) 2-chloro-N-tert-butylacetamide (compound 1a)
[0103] Put tert-butylamine (438.8mg, 6.00mmol) and potassium carbonate (995.1mg, 7.20mmol) in 6mL of dichloromethane solution, and slowly add chloroacetyl chloride (677.6mg, 6.00mmol) dropwise to the above reaction flask under ice-cooling medium, room temperature overnight. After the reaction, an appropriate amount of water was added, extracted with dichloromethane, the combined organic phases were washed with saturated brine, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to obtain a crude product as a white solid. Yield: 74.9%; Melting point: 81.0-82.1°C.
[0104] b) 2-amino-N-tert-butyl-6-methylbenzamide (compound 1b)
[0105] 2-amino-6-methylbenzoic acid (800.0mg, 5.29mmol) was placed in a 50mL single-necked round bottom flask, N 2 After replacement, add...
Embodiment 2
[0121] Example 2: 2-(1-(3-buten-1-yl)-5-methyl-2,4-dione-1,2-dihydroquinazolin-3(4H)-yl)- N-tert-butylacetamide (compound 2)
[0122] a) 1-(3-buten-1-yl)-3-tert-butyl-5-methylquinazoline-2,4(1H,3H)-dione (compound 2a)
[0123] The experimental method was the same as the preparation method of compound 1e in Example 1, except that bromomethylcyclopropane was replaced by 4-bromo-1-butene to obtain a yellow liquid, which was directly used in the next reaction.
[0124] b) 1-(3-buten-1-yl)-5-methylquinazoline-2,4(1H,3H)-dione (compound 2b)
[0125] The experimental method was the same as that of compound 1f in Example 1, except that compound 2a (42.6 mg, 0.15 mmol) was used instead of compound 1f to obtain a white solid. Yield: 42.9%; Melting point: 167.4-168.8°C.
[0126] 1 H NMR (500MHz, CDCl 3 )δ8.42(s, 1H), 7.54(t, J=8.0Hz, 1H), 7.08(d, J=8.5Hz, 1H), 7.05(d, J=7.5Hz, 1H), 5.92-5.84( m, 1H), 5.15-5.10(m, 2H), 4.20-4.16(m, 2H), 2.82(s, 3H), 2.52-2.47(m, 2H).
[0127] c) 2-...
Embodiment 3
[0130] Example 3: N-tert-butyl-2-(5-methyl-2,4-diketone-1-n-propyl-1,2-dihydroquinazolin-3(4H)-yl)acetamide (compound 3)
[0131] a) 3-tert-butyl-5-methyl-1-propylquinazoline-2,4(1H,3H)-dione (compound 3a)
[0132] The experimental method was the same as the preparation method of compound 1e in Example 1, except that bromomethylcyclopropane was replaced by bromo-n-propane to obtain a yellow liquid, which was directly used in the next reaction.
[0133] b) 5-methyl-1-propylquinazoline-2,4(1H,3H)-dione (compound 3b)
[0134] The experimental method was the same as that of compound 1f in Example 1, except that compound 3a (53.8 mg, 0.20 mmol) was used instead of compound 1f to obtain a white solid. Yield: 70.3%; Melting point: 183.8-184.8°C.
[0135] 1 H NMR (500MHz, CDCl 3 )δ8.46(s, 1H), 7.52(t, J=7.5Hz, 1H), 7.07(d, J=8.5Hz, 1H), 7.04(d, J=7.5Hz, 1H), 4.06(t, J=7.5Hz, 2H), 2.82(s, 3H), 1.79-1.75(m, 2H), 1.04(t, J=7.0Hz, 3H).
[0136] c) N-tert-butyl-2-(5-methyl-2,4-dione...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com