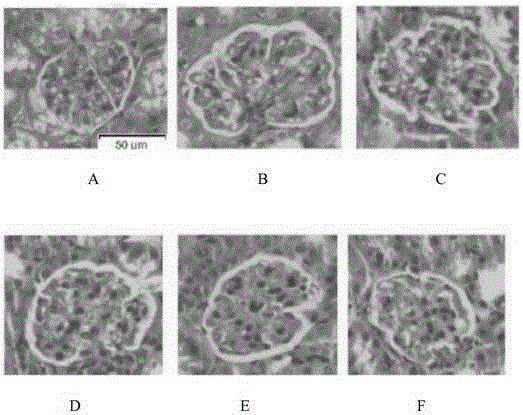
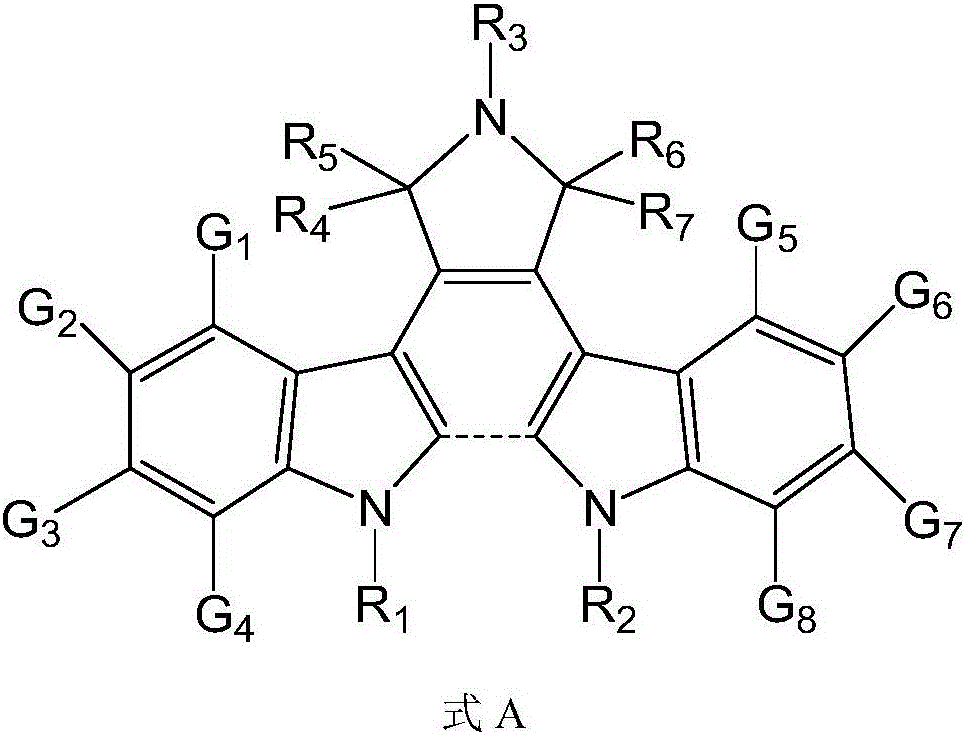
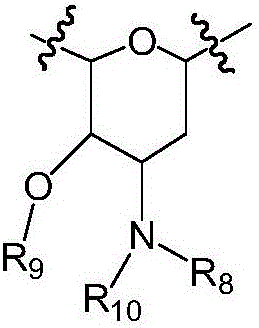
Bisindolyl maleimide derivative and preparation method and application thereof
A compound and alkyl technology, applied in the field of bisindolemaleimide derivatives and their preparation and application, can solve the problems of uncertain curative effect, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] [Example 1] Preparation of Compounds 1-196
[0196] Preparation of Compound 1
[0197] i) Preparation of 1-ethyl-3-indoleacetic acid (1a)
[0198] Under argon protection, add 4g sodium hydride (60% mass fraction, dispersed in paraffin) and 80mL tetrahydrofuran to a 250mL three-necked flask, stir and suspend at 0°C, add 30mL tetrahydrofuran-dissolved indole-3-acetic acid (3.5g, 20mmol), stirred for half an hour, added dropwise 30mL tetrahydrofuran dissolved iodoethane (5mL, 60mmol), slowly raised to room temperature, reacted overnight, lowered to 0°C, added dropwise 10 drops of methanol, and then added an appropriate amount of water to obtain Bright yellow solution, extracted with ethyl acetate, added concentrated hydrochloric acid to the aqueous layer and extracted again, combined the organic layers, and washed with anhydrous Na 2 SO 4 After drying and evaporating to dryness in vacuo, 3.87 g of product (1a) was obtained with a yield of 95.4% after silica gel column c...
Embodiment 2
[0840] [Example 2] activity test
[0841] 1. α-glucosidase inhibitory activity test
[0842] (1) Test method
[0843] Using p-nitrophenyl-α-D-glucopyranoside (PNPG) as substrate, the α-glucosidase inhibitory activity of some compounds was tested. PNPG is an analogue of maltose, which can generate yellow p-nitrophenol after the action of α-glucosidase, which can be directly used for detection by spectrophotometer. Specifically: firstly, the sample was dissolved in PBS sodium phosphate buffer solution (pH6.8) to make 5 concentrations; then 10 μL of sample solution, 20 μL of PBS solution, and 20 μL of 2.5 mM glucose were added to each well of a 96-well plate. Glycoside solution (dissolved in phosphate buffered saline solution), incubate in a 37°C incubator for 5 minutes. Dilute α-glucosidase to 0.2 U / mL with 0.01M PBS solution, add 10 μL from each well to the above test solution, incubate in a 37°C incubator for 15 minutes, and measure the concentration of α-glucosidase at 405...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com