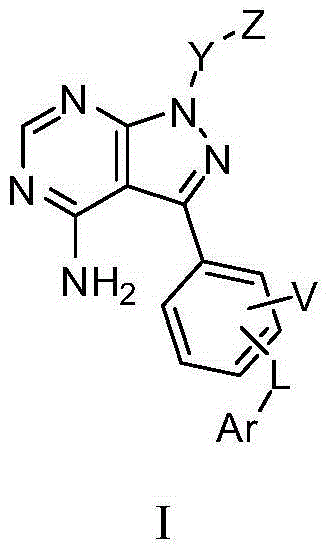
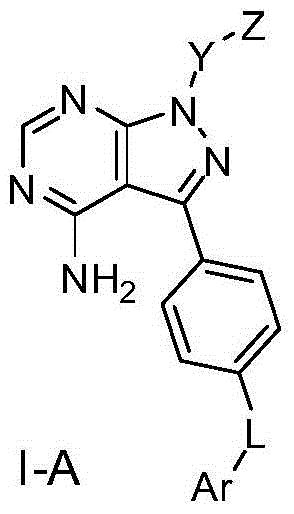
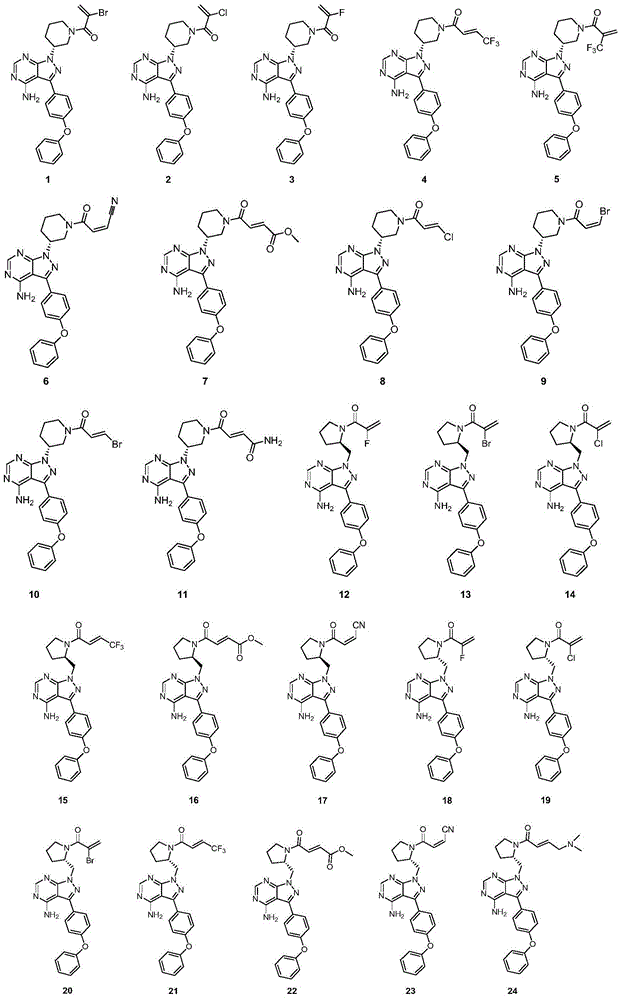
Pyrazolopyrimidine derivative, preparation method, pharmaceutical composition and application
A technology for pyrazolopyrimidine and derivatives, applied in the field of pyrazolopyrimidine derivatives, which can solve the problems of insufficient biological activity and efficacy, large dosage, and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1: (R)-1-[3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine Synthesis of -1-yl]-2-bromoprop-2-en-1-one (compound 1)
[0142]
[0143] Compound 1
[0144] a. Synthesis of (R)-1-Boc-3-(4-amino-3-iodo-1H-pyrazolo[3,4-D]pyrimidin-1-yl)piperidine
[0145]
[0146] 4-Amino-3-iodo-1H-pyrazolo[3,4-D]pyrimidine (10g, 38mmol), (S)-1-Boc-3-hydroxypiperidine (17g, 85mmol), triphenyl Phosphine (20g, 76mmol) was added to a three-necked flask, THF (120ml) was added, the temperature was lowered to 0°C, diisopropyl azodicarboxylate (DIAD) (15.2g, 76mmol) and tetrahydrofuran (THF) (30ml) were added dropwise and mixed solution, the dropwise addition was completed in about 1 h, and slowly rose to room temperature to react overnight. The reaction solution was spin-dried, added water, extracted with ethyl acetate, dried, concentrated and purified by column chromatography to obtain the product (R)-1-Boc-3-(4-amino-3-iodo-1H-pyrazolo[3, 4-D]pyrimidin-...
Embodiment 2
[0160] Example 2: (R)-1-[3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine Synthesis of -1-yl]-2-chloroprop-2-en-1-one (Compound 2)
[0161]
[0162] Compound 2 (72% yield) can be prepared in a similar manner to compound 1, using 2-chloroacrylic acid as a starting material. 1 H-NMR (CDCl 3 , 400MHz, δppm): 8.28(s, 1H), 7.62(d, J=8Hz, 2H), 7.41(t, J=8Hz, 2H), 7.24-7.08(m, 5H), 5.71-5.64(m, 2H),4.98-4.90(m,1H),4.72-4.66(m,0.5H),4.58-4.52(m,0.5H),4.18-4.12(m,0.5H),3.99-3.95(m,0.5H ),3.80-3.78(m,0.5H),3.57-3.52(m,0.5H),3.33-3.28(m,0.5H),2.96-2.90(m,0.5H),2.42-2.25(m,2H) ,2.09-2.01(m,1H),1.85-1.77(m,1H).
Embodiment 3
[0163] Example 3: (R)-1-[3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine Synthesis of -1-yl]-2-fluoroprop-2-en-1-one (Compound 3)
[0164]
[0165] Compound 3 (68% yield) can be prepared in a similar manner to the preparation of compound 1, using 2-fluoroacrylic acid as a starting material. 1 H-NMR (CDCl 3 , 400MHz, δppm): 8.28(s, 1H), 7.62(d, J=8Hz, 2H), 7.41(t, J=8Hz, 2H), 7.24-7.08(m, 5H), 5.71-5.64(m, 2H),4.98-4.90(m,1H),4.72-4.66(m,0.5H),4.58-4.52(m,0.5H),4.18-4.12(m,0.5H),3.99-3.95(m,0.5H ),3.80-3.78(m,0.5H),3.57-3.52(m,0.5H),3.33-3.28(m,0.5H),2.96-2.90(m,0.5H),2.42-2.25(m,2H) ,2.09-2.01(m,1H),1.85-1.77(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com