Pharmaceutical composition for external use
A technology for externally used drugs and compositions, which is applied in the direction of drug combinations, antineoplastic drugs, and pharmaceutical formulations, and can solve the problems that there are no reports on the combined use of timolol and allantoin, so as to overcome the problems of adverse reactions, Increased transdermal absorption, good soothing and soothing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
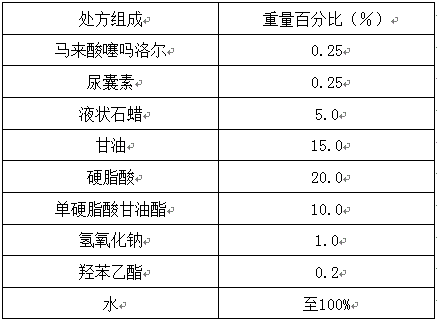
[0047]
[0048] Preparation process: Heat the oily and oil-soluble components (liquid paraffin, stearic acid, glyceryl monostearate) in the prescription together to about 80°C to form an oil solution (oil phase), and add the water-soluble components (glycerin , sodium hydroxide) dissolved in water and heated together to 80 ° C to form an aqueous solution (water phase). When the oil phase is mixed with the water phase, add the water phase to the oil phase. After stirring for 30 minutes, add timolol maleate, allantoin and ethylparaben in sequence, continue stirring for 30 minutes, and cool to room temperature under stirring to obtain timolol maleate cream.
Embodiment 2
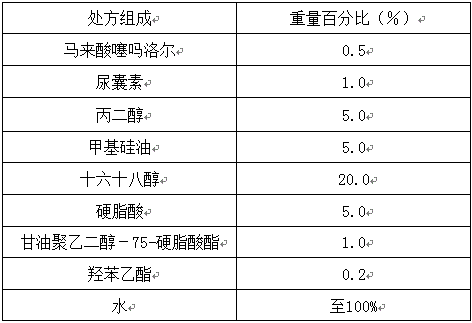
[0050]
[0051] Preparation process: Heat the oily and oil-soluble components (methicone, stearic acid, cetostearyl alcohol) in the prescription together to about 75°C to form an oil solution (oil phase), and add the water-soluble components (horse Timolol toate, propylene glycol, allantoin, glycerol macrogol-75-stearate, ethylparaben) are dissolved in water and heated together to 70°C to form an aqueous solution (water phase). When the oil phase is mixed with the water phase, stir for 30 minutes, and then cool to room temperature under stirring to obtain timolol maleate cream.
Embodiment 3
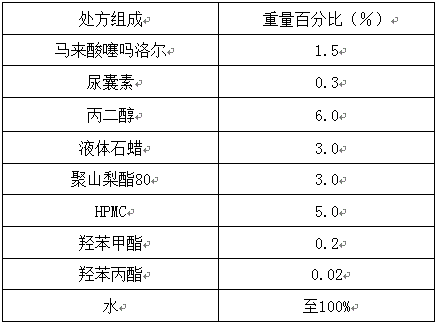
[0053]
[0054] Preparation process: Heat the oily and oil-soluble components (liquid paraffin) in the prescription to about 30°C to form an oil solution (oil phase), and add water-soluble components (HPMC, timolol maleate, propylene glycol, urine Blastosin, polysorbate 80, methylparaben / propyl ester) are dissolved in water and heated together to 30°C to form an aqueous solution (water phase). The oil phase is mixed with the water phase, and after stirring for 30 minutes, timolol maleate cream is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com