Method for catalyzing cross coupling reaction of Suzuki with palladium magnesium aluminum class layered double hydroxides (LDHs)
A cross-coupling reaction, hydrotalcite technology, applied in chemical instruments and methods, preparation of organic compounds, catalyst regeneration/reactivation, etc., can solve the problems of poor palladium stability, end products and environmental pollution, and achieve high production efficiency , reduce pollution and distribute evenly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of catalyst PdMgAl-LDH-1 (0.50% Pd, w / w):
[0041] Weigh Mg(NO 3 ) 2 ·6H 2 O (4.590g, 17.90mmol), A1 (NO 3 ) 3 9H 2 O(2.251g, 6.00mmol), Pd(NO 3 ) 2 ·6H 2 O (0.027g, 0.10mmol), HNO 3 (0.10mol / L, 1mL) was dissolved in 12.0mL deionized water to form a mixed salt solution (A); NaOH (1.540g, 38.50mmol), NaOH 2 CO 3 (1.272g, 12.00mmol) was dissolved in 12.0mL of deionized water to prepare an alkaline solution (B). The two solutions (A) and (B) were slowly added dropwise into a three-necked round-bottomed flask filled with 9.0 mL of deionized water at the same time, vigorously stirred, and the dropping speed was controlled to maintain pH=9.5. After the dropwise addition, age in a water bath at 100°C for 13 hours, filter, wash the solid with water until neutral, and dry at 110°C for 24 hours to obtain 2.012 g of a gray solid, which is finely ground and sieved for later use.
Embodiment 2
[0043] Preparation of catalyst PdMgAl-LDH-2 (2.58% Pd, w / w):
[0044] Weigh Mg(NO 3 ) 2 ·6H 2 O (4.487g, 17.50mmol), A1 (NO 3 ) 3 9H 2 O(2.251g, 6.00mmol), Pd(NO 3 ) 2 ·6H 2 O (0.133g, 0.50mmol), HNO 3 (0.10mol / L, 1.0mL) configuration salt solution (A); NaOH (1.540g, 38.50mmol), NaOH 2 CO 3 (1.272g, 12.00mmol) Alkaline solution (B) was prepared, and the subsequent steps were the same as in Example 1 to obtain 2.012g of gray powder.
Embodiment 3
[0046] Catalyst PdMgAl-LDH-1 regeneration to prepare PdMgAl-LDH-3:
[0047] First, the deactivated catalyst PdMgAl-LDH-1 (2.000g, 0.46%Pd, w / w) was washed with hot ethanol to remove organic residues after 10 reactions (see Example 4), and then the catalyst was washed with 11.0mL 20 % nitric acid nitrate, to obtain a clear solution (A); NaOH (3.200g, 80.00mmol), Na 2 CO 3 (1.272g, 12.00mmol) Alkaline solution (B) was prepared. Subsequent steps were the same as in Example 1 to obtain 1.966 g of PdMgAl-LDH-3 catalyst, gray powder containing 0.44% Pd (w / w).
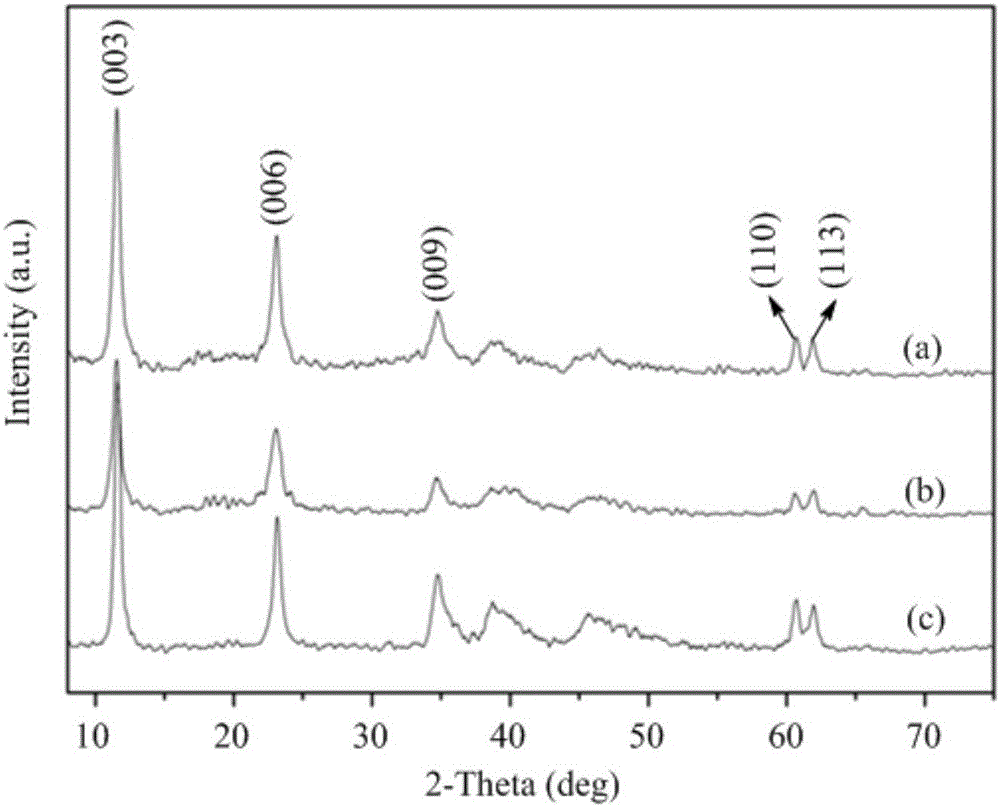
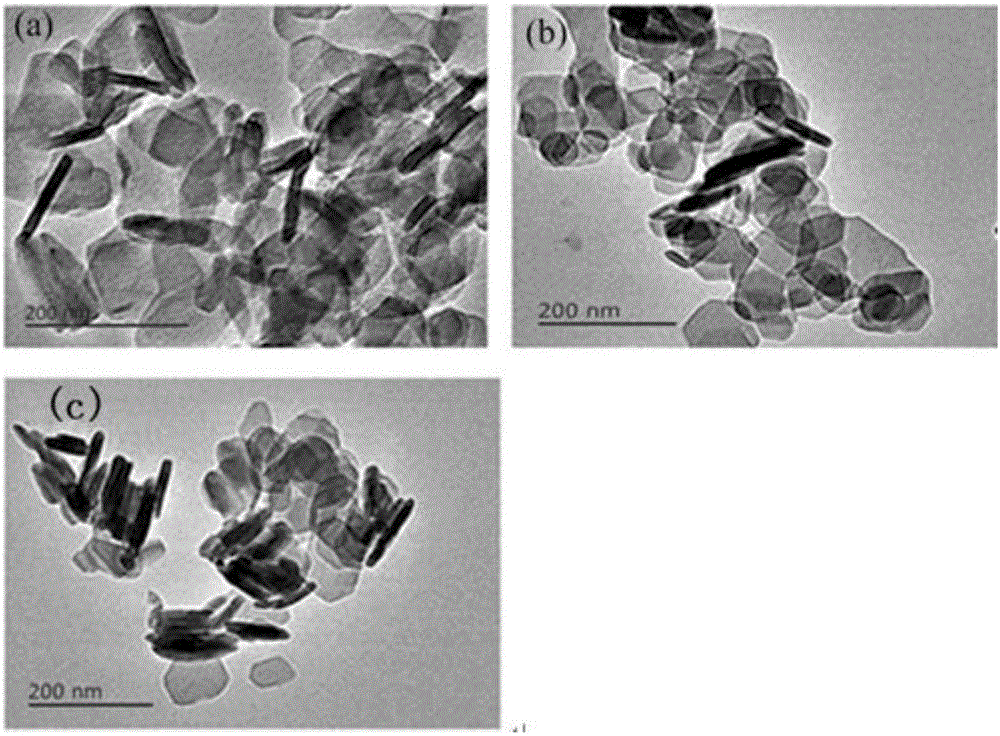
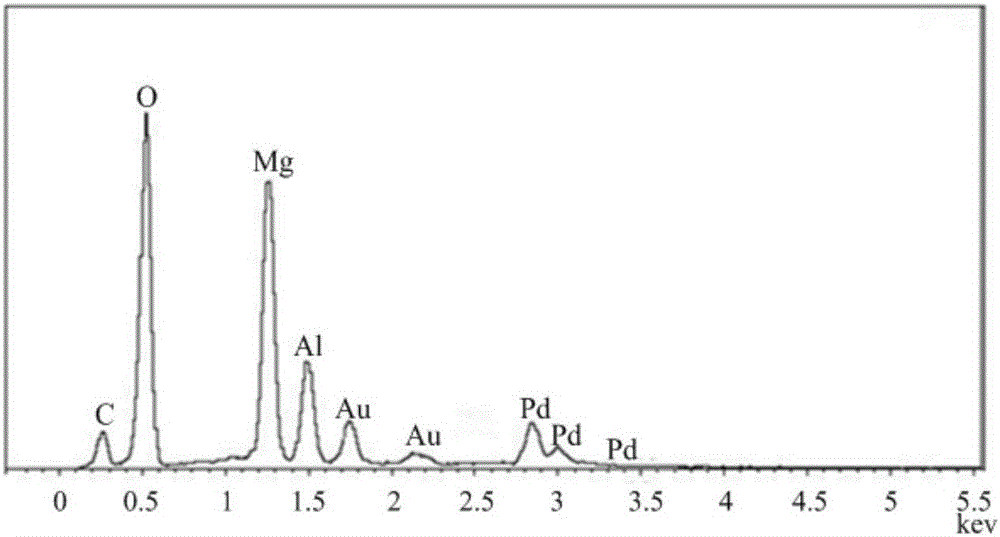
[0048] From the XRD spectrum of PdMgAl-LDH ( figure 1 ) It can be seen that around 2θ=11.6, 23.2, 34.8, 60.5 and 62.0°, there are characteristic diffraction peaks of hydrotalcite. Compared with the standard spectrum of LDHs (JCPDS 51-1525), the intensity of each diffraction peak of XRD is large, and the peak shape is sharp. Good symmetry indicates that the prepared and regenerated catalyst has good crystallinity; no other...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com