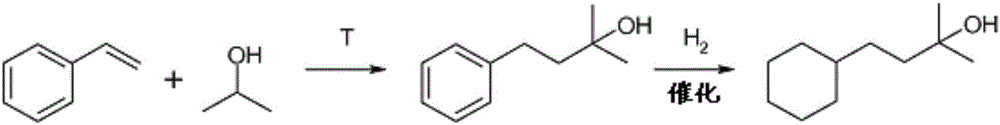
Method for producing 4-cyclohexyl-2-methyl-2-butanol
A technology of cyclohexyl and methyl, which is applied in the field of preparing 4-cyclohexyl-2-methyl-2-butanol, can solve the problems of undescribed preparation methods, achieve high selectivity and prevent movement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0106] Preparation Example 1: Preparation of Hydrogenation Catalyst
[0107] The support material used is spherical SiO 2 Carrier (type AF125 from BASF SE) with a ball diameter of 3-5 mm and a bulk density of 0.49 kg / l. BET surface area is 337m 2 / g, water absorption (WA) is 0.83ml / g. For impregnation a 14.25% by weight solution of ruthenium(III) acetate in acetic acid from Umicore was used.
[0108] 200 g of the carrier was first added to the round bottom flask. 15 g of ruthenium acetate solution was diluted to 150 ml (90% WA) with distilled water. The support material was first charged into the distillation flask of the rotary evaporator and the first quarter of the solution was pumped onto the support material at 3-6 rpm under slightly reduced pressure. When the addition was complete, the support was left on the rotary evaporator for an additional 10 minutes at 3-6 rpm to homogenize the catalyst. This impregnation-homogenization step is repeated three more times until...
Embodiment 1
[0110] Step a)
[0111] The reaction was carried out in a continuous laboratory apparatus as a reactor containing a 300 ml autoclave, which was operated under pressure regulation. Thus, the amount withdrawn at any time corresponds to the amount introduced. The withdrawn reaction mixture was cooled, decompressed, and collected in a discharge vessel.
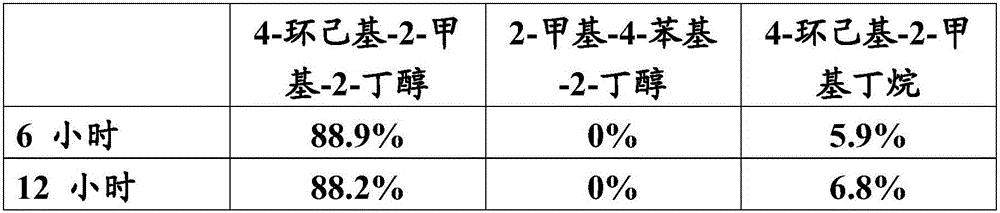
[0112] A solution of styrene in isopropanol (30% by weight, 200 g / h) was pumped in the reactor via laboratory equipment at an average temperature of 410°C. The conversion of styrene was 85.1%, and 12.5 g / h of 2-methyl-4-phenyl-2-butanol were obtained at steady state. Samples were analyzed by gas chromatography.
[0113] Step b)
[0114] In a 300ml autoclave, first add 10.2g of 2-methyl-4-phenyl-2-butanol (62mmol) from step a) dissolved in 150ml of tetrahydrofuran (62mmol), and the catalyst basket from Preparation Example 1 1.7g of catalyst. The autoclave was purged three times with nitrogen and then hydrogen was injected to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical temperature | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com