Method for synthesizing oxime ether compound based on C-N bond breakage
A synthesis method and compound technology are applied in the fields of pharmaceutical chemical intermediates and related chemistry to achieve the effects of wide substrate range, easy product and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
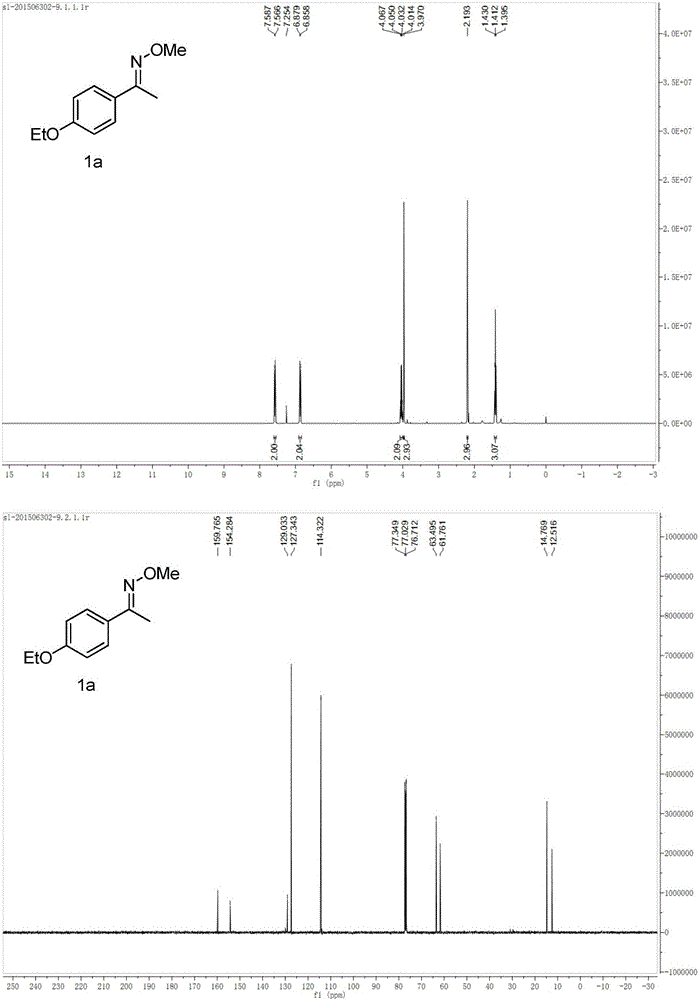
[0034] Example 1: Synthesis of 1-(4-ethoxyphenyl)ethanone O-methyl oxime (1a)
[0035]
[0036] Add p-ethoxyacetophenone (0.75mmol, 127mg), N-methoxybenzamide (0.5mmol, 78.2mg), potassium persulfate (1.0mmol, 270.3mg), anhydrous methanol 2mL into the reactor , sealed, and stirred at 80°C for 20 hours. Cool to room temperature, remove the solvent under reduced pressure, separate and purify by column chromatography (eluent: petroleum ether / ethyl acetate=600:1) to obtain 90.8 mg of 1-(4-ethoxyphenyl)ethanone O-methyl oxime (1a) , yield 94%.
[0037] 1 H NMR (400MHz, CDCl 3 ): δ7.58(d, J=8.5Hz, 2H), 6.87(d, J=8.5Hz, 2H), 4.04(q, J=7.0Hz, 2H), 3.97(s, 3H), 2.19(s ,3H),1.41(t,J=7.0Hz,3H); 13 C NMR (100MHz, CDCl 3 ): δ159.8, 154.3, 129.0, 127.3, 114.3, 63.5, 61.8, 14.8, 12.5.
Embodiment 2
[0038] Example 2: Synthesis of 1-(4-ethoxyphenyl)ethanone O-methyl oxime (1a)
[0039]
[0040] Add p-ethoxyacetophenone (0.75mmol, 127mg), N-methoxybenzamide (0.5mmol, 78.2mg), sodium persulfate (1.0mmol, 238.1mg), anhydrous methanol 2mL into the reactor , sealed, and stirred at 80°C for 20 hours. Cool to room temperature, remove the solvent under reduced pressure, and separate and purify by column chromatography (eluent: petroleum ether / ethyl acetate=600:1) to obtain 45.1 mg of 1-(4-ethoxyphenyl)ethanone O-methyl oxime (1a) , yield 47%.
Embodiment 3
[0041] Example 3: Synthesis of 1-(4-ethoxyphenyl)ethanone O-methyl oxime (1a)
[0042]
[0043]Add p-ethoxyacetophenone (0.75mmol, 127mg), N-methoxybenzamide (0.5mmol, 78.2mg), potassium persulfate (1.0mmol, 270.3mg), absolute ethanol 2mL into the reactor , sealed, and stirred at 80°C for 20 hours. Cool to room temperature, remove the solvent under reduced pressure, and separate and purify by column chromatography (eluent: petroleum ether / ethyl acetate=600:1) to obtain 26.1 mg of 1-(4-ethoxyphenyl)ethanone O-methyl oxime (1a) , yield 27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com