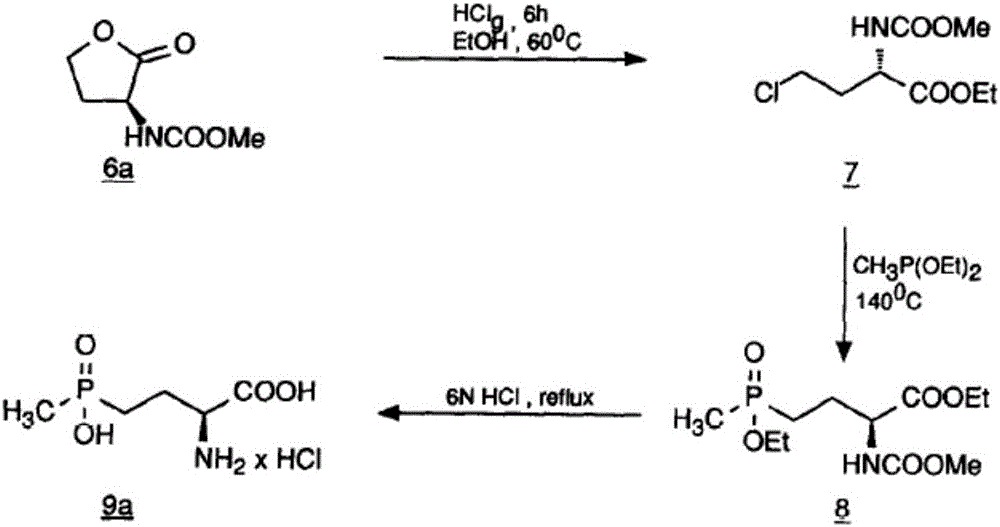
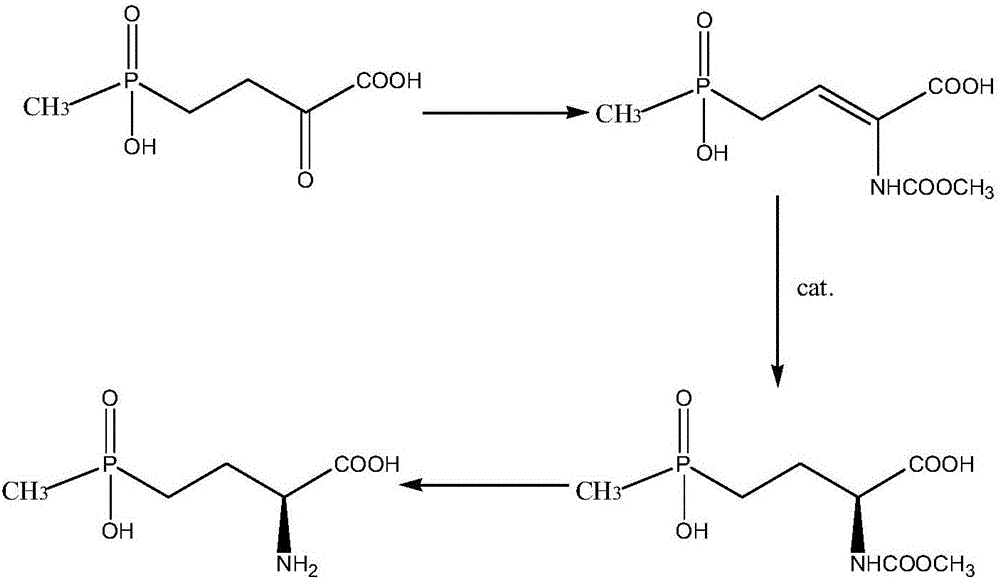
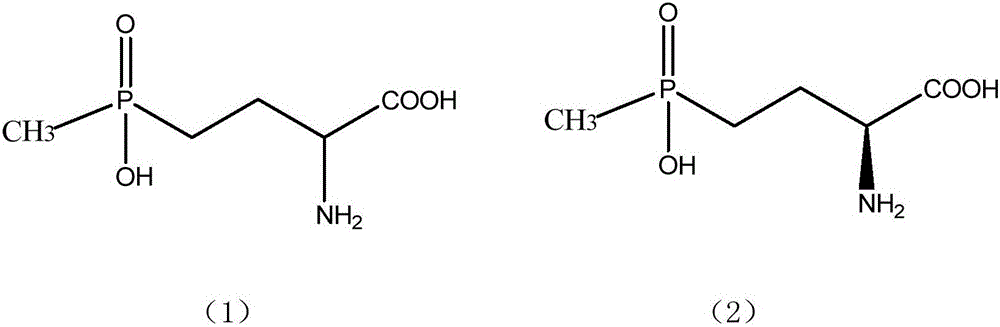
Method for separation and refining of L-glufosinate-ammonium and salts thereof
A purification method and glufosinate-ammonium technology are applied in the field of separation and purification of L-form and D-form, which can solve the problems of high cost, residue, complicated separation of chiral isomers, etc., and achieve the effect of solving difficult purification and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 200ml of water into a 500ml three-necked bottle, and add hydrochloric acid dropwise to adjust the pH to 1.9. Add 100 grams of L-body glufosinate-ammonium crude product (L:D=92.7:7.3), heat up to 45°C, and keep warm at 45-50°C for 2 hours.
[0041] Cool down to normal temperature (20°C), filter, and separate to obtain a low-L body component solid, which weighs 18 grams after drying, and the measured L:D is 66:34; the filtrate is concentrated and dehydrated, and 800ml of methanol is added, stirred, cooled, filtered, and dried Weighing 81.5 grams, analysis L:D=98.5:1.5.
Embodiment 2
[0043] Add 150ml of water into a 500ml three-necked flask, and add sulfuric acid dropwise to adjust the pH to 1.9. Add 100 g of crude L-glufosinate-ammonium (L:D=86.0:14.0), raise the temperature to 45° C., and keep stirring for 2 hours.
[0044] Cool down to normal temperature, filter and separate to obtain the low-L body component solid, dry weight 34.5 grams, measure L:D=65:35; concentrate and dehydrate the filtrate, add 700ml of methanol, stir, cool down, filter to obtain 65.0 grams of solid, measure L: D's result was 97:3.
Embodiment 3
[0046] Add 32 grams (L: D = 65: 35) of the crude product obtained from one separation into a 500 ml three-necked flask, add 60 ml of acidic water with pH = 1.9, repeat the operation as in Example 1 to separate, and dehydrate the filtrate with methanol After treatment, 21.0 g of the racemate solid was obtained by separation, and the measured L:D=50.5:49.5; 10.8 g of the L-rich component was obtained, and the measured L:D result was 92.5:7.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com