Evaluation method related to hydrolysis reaction activity of neonicotinoid insecticides
A technology of neonicotinoids and hydrolysis reaction, applied in the direction of chemical method analysis, measuring devices, instruments, etc., can solve the problems of cumbersome connection of hydrolysis activity, etc., and achieve high practical value, accurate and reliable results, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Evaluation of Thiacloprid and Acetamiprid Hydrolysis Reactivity
[0025] The neonicotinoid insecticides to be evaluated are thiacloprid and acetamiprid. Thiacloprid and acetamiprid have the same saturated heterocycle and cyanimide group, the difference is that thiacloprid is a ring-closing cyanimide functional group, and acetamiprid is a ring-opening cyanimide functional group. The evaluation method is as follows:
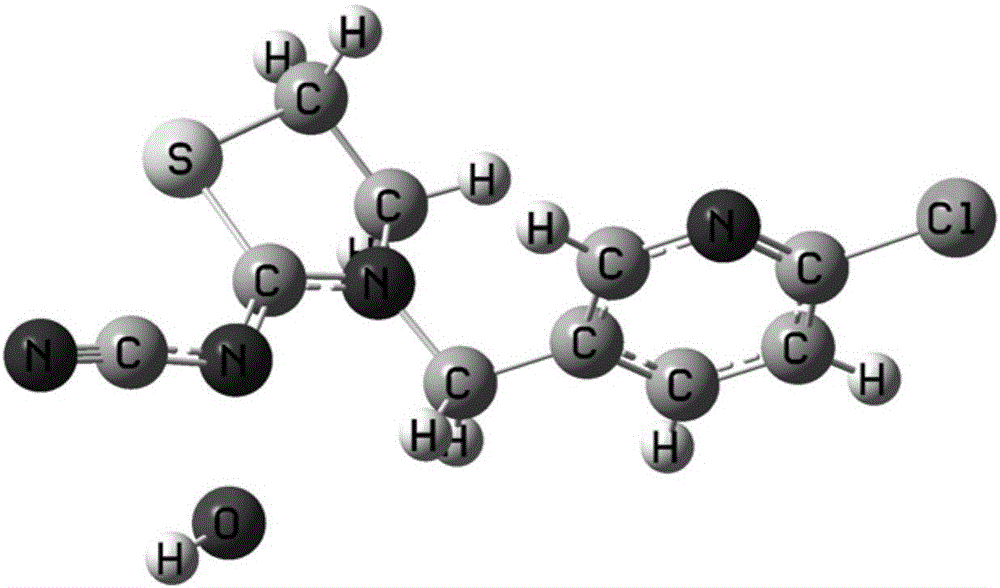
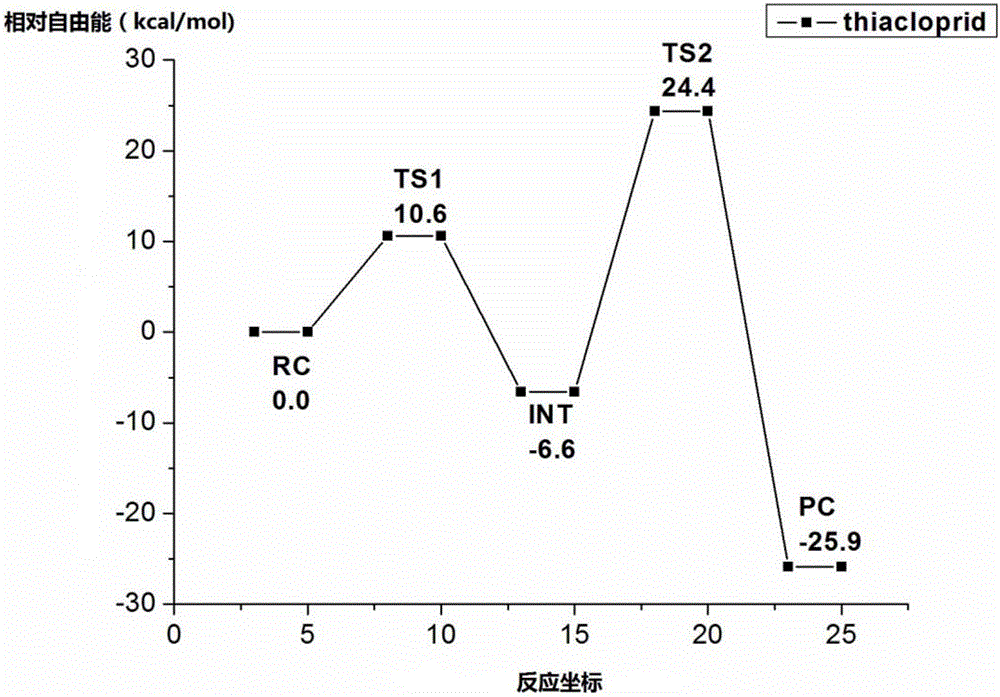
[0026] The first step is to establish the calculation model of the hydrolysis reaction of thiacloprid molecule: obtain the X-ray diffraction crystal structure of thiacloprid molecule from the database PubChem, and insert water molecules at its active site. Using the density functional B3LYP method in the Gaussian 03 program and the medium-sized polarized dispersive basis set 6-31+G(d,p) to optimize its structure, the calculation model of the hydrolysis reaction of thiacloprid molecules is obtained, as shown in figure 1 shown.
[0027] According ...
Embodiment 2
[0032] Example 2 Evaluation of Thiacloprid and Imidacloprid Hydrolysis Reactivity
[0033] The neonicotinoid insecticides to be evaluated are thiacloprid and imidacloprid. Thiacloprid and imidacloprid have the same saturated heterocycle, but the difference is that thiacloprid is a ring-closing cyanoimide functional group, and imidacloprid is a ring-closing nitroguanidine functional group. The evaluation method is as follows:
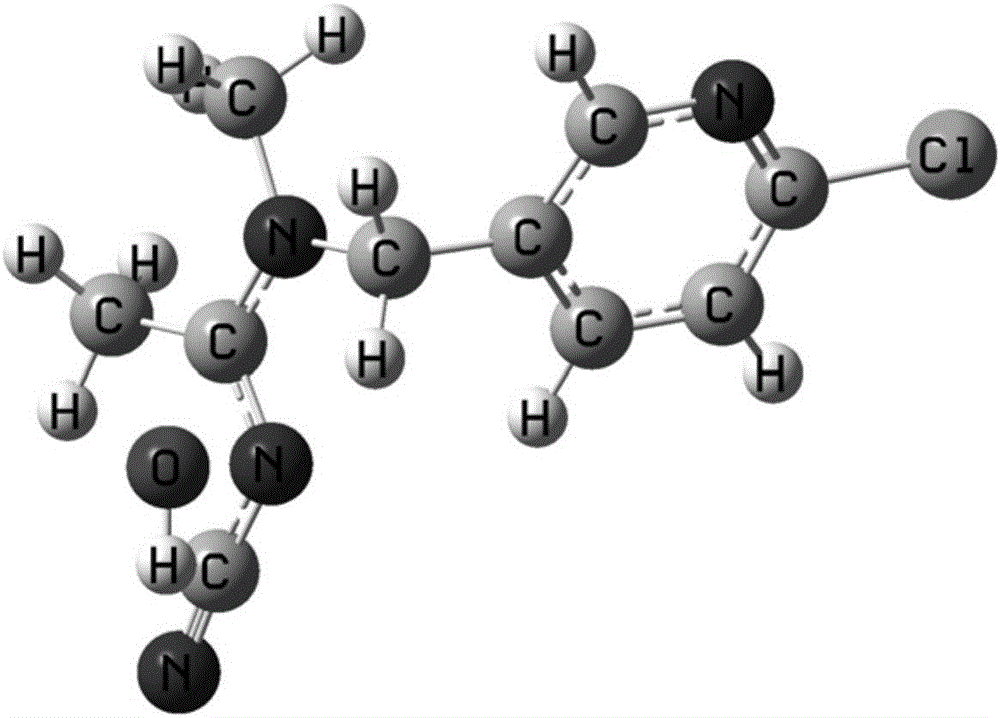
[0034] The first step is to establish the calculation model of the hydrolysis reaction of thiacloprid molecule: obtain the X-ray diffraction crystal structure of thiacloprid molecule from the database PubChem, and insert water molecules at its active site. Using the density functional B3LYP method in the Gaussian 03 program and the medium-sized polarized dispersive basis set 6-31+G(d,p) to pre-optimize its structure, the calculation model of the hydrolysis reaction of thiacloprid was obtained.
[0035] According to the calculation model of the hydrolys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com