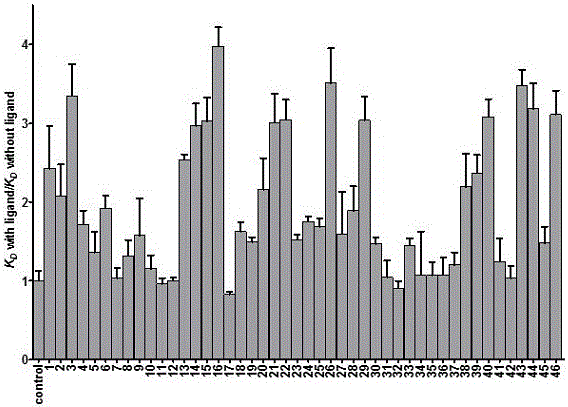
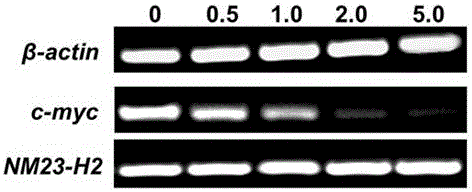
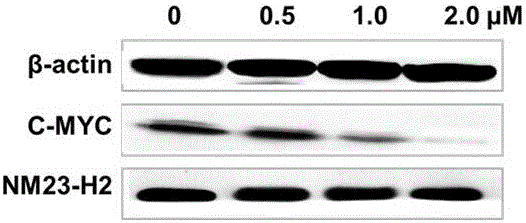
7-fluoro-substituted Isaindigotone derivatives and preparation method thereof, and application of 7-fluoro-substituted Isaindigotone derivatives in preparing anticancer drugs
A derivative and hydroxyl technology, applied in the 7-position fluorine substituted Isaindigotone derivative and its preparation, the application field in the preparation of anti-cancer drugs, to achieve the effect of inhibiting proliferation and widely anti-tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: the synthesis of compound m1
[0078]
[0079] Put 2-amino-4,5-difluorobenzoic acid (5g, 29mmol) and 2-pyrrolidone (5mL) into a 100mL single-necked flask, and add 20mL of phosphorus oxychloride (POCl3) dropwise under stirring in an ice bath to make After 30 minutes of dripping, heat and reflux at 103°C for 24 hours, add the reaction system dropwise to 200mL of ice water, adjust the pH to weak alkaline with concentrated NaOH solution, and filter with suction to obtain the crude product, which is purified by silica gel column chromatography (eluent: V (Petroleum ether): V (ethyl acetate) = 1:1) to obtain compound m1, 5.4 g, yield 85%.
[0080] 1 H NMR (400MHz, CDCl 3 )δ8.20–7.85(m,1H),7.60–7.34(m,1H),4.42–4.06(m,2H),3.19(td,J=7.9,2.7Hz,2H),2.43–2.16(m, 2H).LC-MS m / z:223[M+H] +.
Embodiment 2
[0081] Embodiment 2: the synthesis of compound m2
[0082]
[0083] Take compound m1 (2.22g, 10mmol) and diethylaminopropylamine (3ml, 24mmol) in a thick-walled pressure-resistant bottle, heat and stir at 100°C overnight to obtain an orange-yellow solution. After the reaction system cools down to room temperature, a large amount of pale A yellow precipitate was precipitated, and 20 ml of diethyl ether was added to the reaction system to further precipitate the solid. After suction filtration, the filter cake was obtained as a milky white solid, which was vacuum-dried to obtain 2.3 g of milky white powder, with a yield of 69%.
[0084] 1 H NMR (400MHz, CDCl 3 )δ7.72(d,J=11.7Hz,1H),6.72(s,1H),6.67(d,J=7.7Hz,1H),4.19–4.12(m,2H),3.31(dd,J=10.6 ,5.8Hz,2H),3.11(t,J=7.9Hz,2H),2.63–2.59(m,2H),2.55(q,J=7.1Hz,4H),2.30–2.19(m,2H),1.89 –1.81(m,2H),1.06(t,J=7.1Hz,6H).
Embodiment 3
[0085] Embodiment 3: the synthesis of compound m3
[0086]
[0087] According to the synthesis method of m2, 2.1 g of light yellow solid was obtained with a yield of 66%. 1 H NMR (400MHz, CDCl 3 )δ7.67(d,J=11.7Hz,1H),6.63(d,J=7.7Hz,1H),4.12–4.05(m,2H),3.24(dd,J=11.6,6.3Hz,2H), 3.04(t, J=7.9Hz, 2H), 2.37(t, J=6.4Hz, 4H), 2.19(s, 6H), 1.77(dt, J=12.8, 6.2Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com