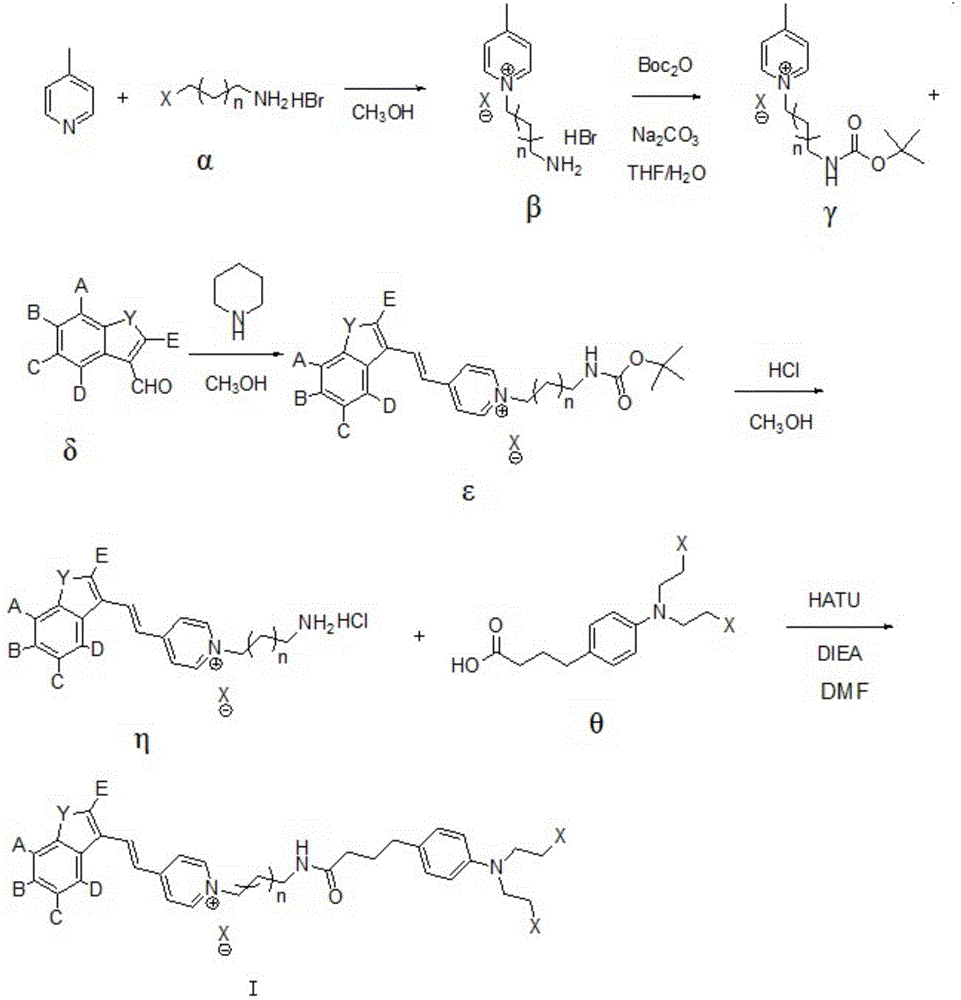
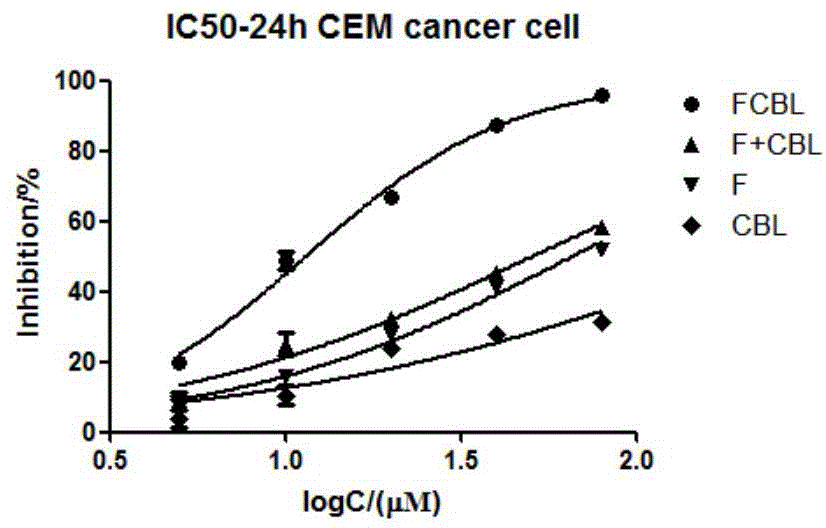
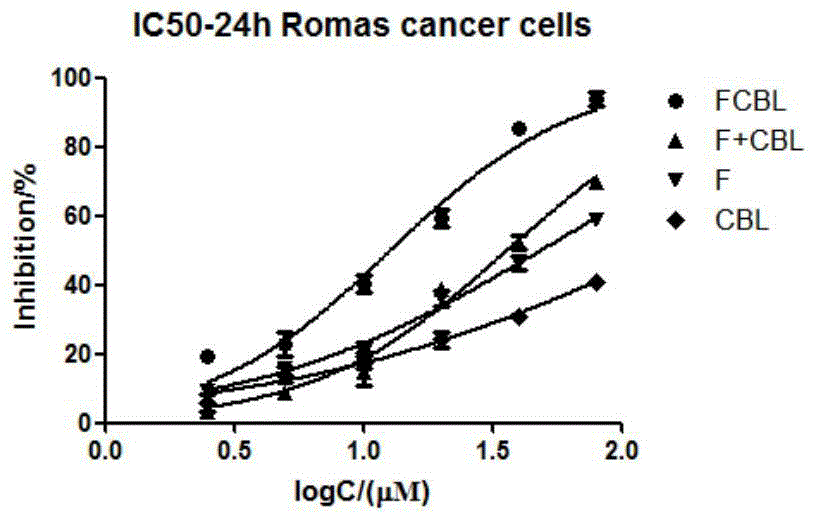
Compound synthesized from targeting antitumor drug F16 and chlorambucil, and preparation method thereof
An anti-tumor drug, chlorambucil technology, applied in anti-tumor drugs, drug combinations, organic chemistry, etc., can solve the problems of multi-drug resistance and low selectivity of anti-cancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] First, get compound 1 (3-bromopropylamine hydrobromide) and 4-picoline reaction, compound 1 structure is as follows:
[0081]
[0082] During specific implementation, respectively add 4-picoline (2g), 3-bromopropylamine hydrobromide (1.0eq), 10ml of anhydrous methanol (or absolute ethanol, anhydrous ether) in the reaction flask, then heat to reflux React overnight. After the reaction was complete, the reaction system was cooled to room temperature, filtered, washed with a small amount of methanol, and dried to obtain a white solid, namely compound 2 (5.0 g); in this example, the structure of compound 2 was as follows:
[0083]
[0084] Next, add compound 2 (2.27g), 20ml of tetrahydrofuran, 20ml of anhydrous sodium carbonate (2.0eq) dissolved in water, stir at room temperature for 30min, then slowly add di-tert-butyl dicarbonate (1.1eq) dropwise into the reaction flask In 10ml of tetrahydrofuran, react at 25-30°C for 4 hours. Filtrate, spin the filtrate to drynes...
Embodiment 2
[0097] The method for preparing compound 3 is the same as that in Example 1, and will not be repeated here.
[0098] Get compound 3 and compound 9 (6-fluoroindole-3-formaldehyde) reaction,
[0099]
[0100] The specific steps are: respectively add compound 3 (1.0g), 6-fluoroindole-3-carbaldehyde (1.0eq), and anhydrous methanol 30ml into the reaction flask, then add piperidine (0.9eq), and heat up to 50°C After the reaction was completed overnight, the reaction system was spun dry and passed through the column, then an appropriate volume of dichloromethane and methanol (35:1) was added for stirring, and then filtered, and the filtrate was spun dry again to obtain a deep red semi-solid compound 10 (0.7g), the structure of compound 10 is as follows:
[0101]
[0102]
[0103] Then, compound 10 (0.5 g), 5 ml of methanol (or ethanol, ether) were added into the reaction flask, 2.5 ml of concentrated hydrochloric acid was added dropwise at room temperature, and the reaction...
Embodiment 3
[0108] The method of preparing compound 3 is the same as that in Example 1, and will not be repeated here.
[0109] Get compound 3 and compound 13 (5-fluoroindole-3-formaldehyde) reaction,
[0110]
[0111] The specific steps are: respectively add compound 3 (1.0g), 5-fluoroindole-3-carbaldehyde (1.0eq), and anhydrous methanol 30ml into the reaction flask, then add piperidine (0.9eq), and heat up to 50°C React overnight. The reaction was complete, the reaction system was spin-dried and passed through the column, then an appropriate volume of dichloromethane and methanol (25:1) was added to stir, and then filtered, and the filtrate was spin-dried again to obtain a dark red semi-solid compound 14 (0.75g ), the structure of compound 14 is shown below:
[0112]
[0113] Subsequently, compound 14 (0.5 g) and 5 ml of methanol were added to the reaction flask, 2.5 ml of concentrated hydrochloric acid was added dropwise at room temperature, and reacted at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com