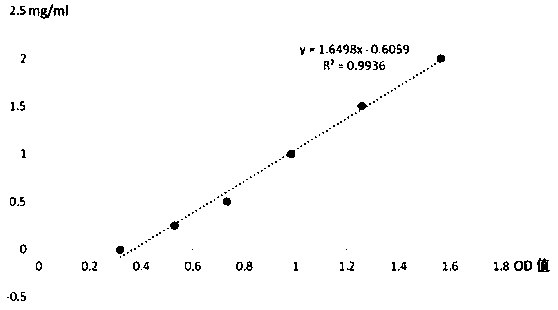
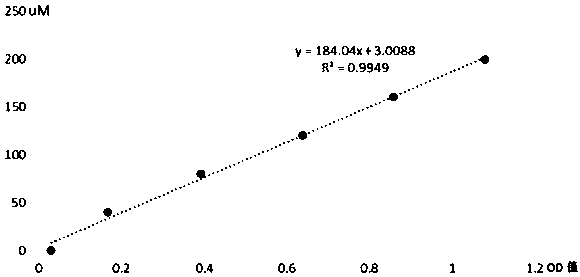
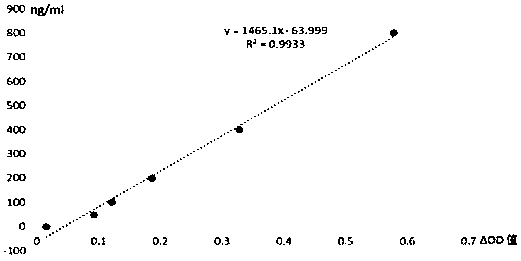Method for measuring mpo halogenase activity
A technology of halogenase activity and halogenase, which is applied in the field of natural protease purification, can solve the problems of long time, long time required, high preparation cost, etc., and achieve the effect of strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0069] The step that the MPO halogenase measured by the present invention is separated from HL-60 cells is:
[0070] a. Isolate MPO from HL-60 cells: culture HL-60 cells, collect the cells by centrifugation, lyse the cells with phosphate buffer (pH8.0), and then use Dounce homogenization to break the cells, centrifuge, and collect the cell sediments. Cell sediments contain MPO;
[0071] b. 1% CTAB (Cetyltrimethylammonium bromide)-phosphate buffer (pH8.0) dissolves MPO in the above cell deposits;
[0072] c. Preparation of Q-Sepharose chromatography column: Equilibrate the Q-Sepharose chromatography column with 0.5% CTAB-phosphate buffer (pH8.0), the above MPO solution passes through the Q-Sepharose chromatography column, and the binding protein (pI≤8.0 ) onto the Q-Sepharose chromatography column, and collect the filtrate (containing MPO, pI=9.2);
[0073] d. Preparation of SP-Sepharose chromatography column: Equilibrate the SP-Sepharose chromatography column with 0.2% CTAB-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com