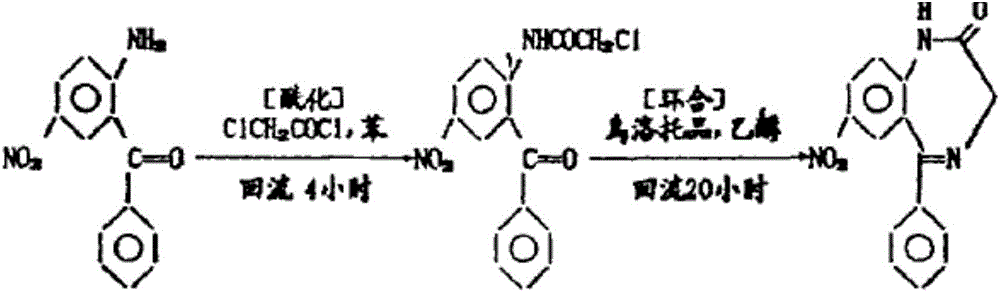
Preparation method of 2-chloracetylamino-5-nitro benzophenone
A technology of nitrobenzophenone and chloroacetamido is applied in the field of preparation of 2-chloroacetamido-5 nitrobenzophenone, and can solve the problem of not meeting British Pharmacopoeia standards, the product containing many impurities, and the inability to export, etc. problem, to achieve the effect of reasonable design of process steps, high purity and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
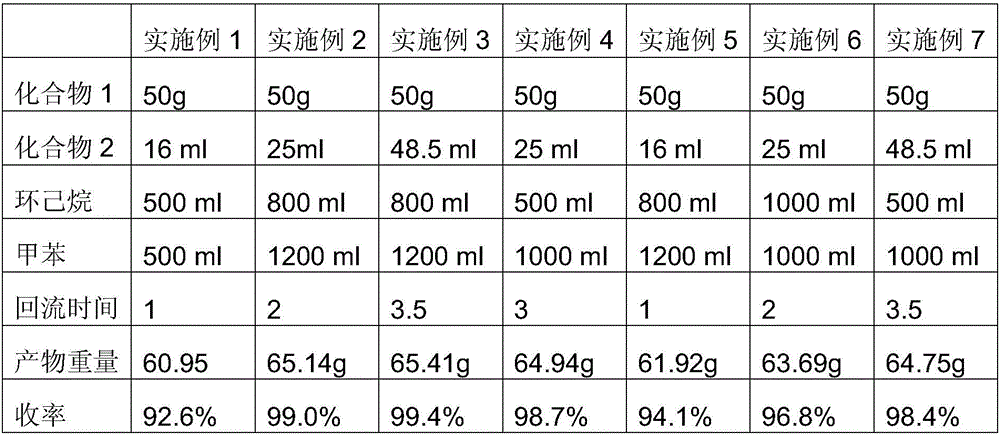
[0016] Add 2-amino-5-nitro-benzophenone into the mixed solvent of cyclohexane and toluene, heat up to reflux, cool down slightly until the reflux stops, add chloroacetyl chloride dropwise under stirring, and reflux for 1 to 3.5 hours after the addition . Then cool down to room temperature, filter, wash with water until neutral, filter and dry to obtain 2-chloroacetamido-5-nitro-benzophenone. The specific experimental conditions and results of each embodiment are shown in Table 1.
[0017] Table 1
[0018]
[0019]
[0020] Note: Compound 1 is 2-amino-5-nitro-benzophenone;
[0021] Compound 2 is chloroacetyl chloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com