Method for preparing copper-based catalyst for catalyzing carbon dioxide hydrogenation reduction
A technology for catalyzing carbon dioxide and copper-based catalysts, which is applied in physical/chemical process catalysts, chemical instruments and methods, chemical elements of heterogeneous catalysts, etc. The effect of small size and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of CuO / ZnO catalyst
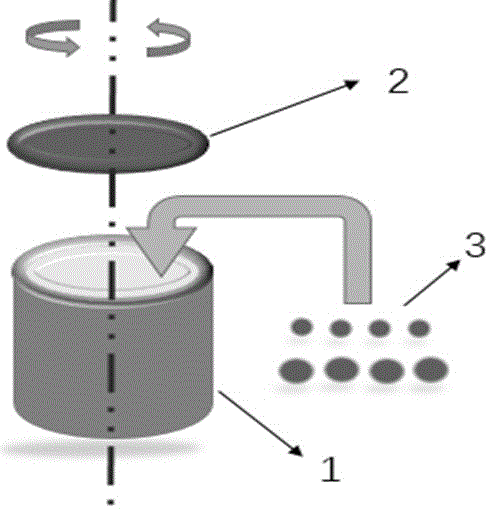
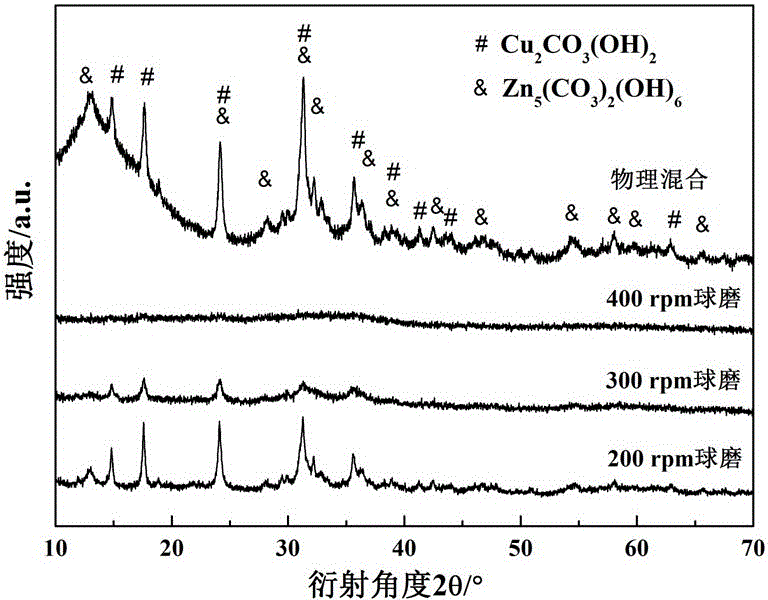
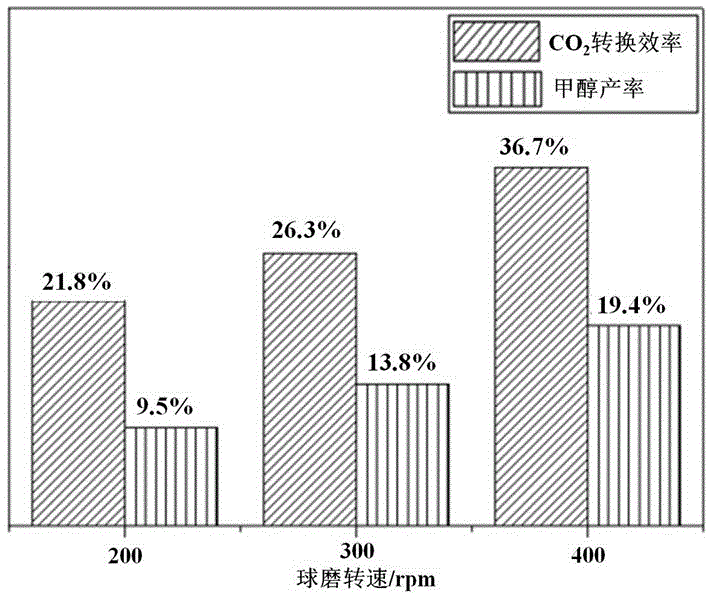
[0035] Basic copper carbonate and basic zinc carbonate are compared according to the copper-zinc ion ratio Cu 2+ :Zn 2+ =7:3 Weigh it and add it to the ball mill tank 1, and add stainless steel balls according to the mass ratio of balls to material at 15:1. Then add an appropriate amount of acetone to the ball mill jar to make the powder sample into a paste, load the ball mill jar on the ball mill, and ball mill for 20 h (hours) at 200 rpm (rotation per minute), 300 rpm and 400 rpm respectively, where Stop once every 2 h, each stop 6 min (minutes). After ball milling, the steel balls were separated from the sample and the catalyst precursor was collected after the acetone volatilized. The obtained catalyst precursor was calcined at 320 °C for 3 h, and a black CuO / ZnO catalyst was obtained after the end. The XRD patterns of CuO / ZnO catalyst precursors synthesized at different ball mill speeds are as follows figure 2 shown.
[...
Embodiment 2
[0039] (1) Preparation of CuO / ZnO catalyst
[0040] Basic copper carbonate, basic zinc carbonate and zirconium oxide according to Cu 2+ :Zn 2+ :Zr 4+=2:1 Weigh it and add it to the ball mill tank 1, and add stainless steel balls according to the mass ratio of balls to material at 20:1. Then add an appropriate amount of acetone to the ball milling tank to make the powder sample into a paste, load the ball milling tank on the ball mill, and ball mill at 200 rpm, 300 rpm and 400 rpm for 30 h respectively, stop every 2 h, Stop for 10 minutes each time. After ball milling, the steel balls were separated from the sample and the catalyst precursor was collected after the acetone volatilized. The obtained catalyst precursor was calcined at 310 °C for 3 h, and a black CuO / ZnO catalyst was obtained after the end.
[0041] (2) Performance characterization of CuO / ZnO catalyst
[0042] The CuO / ZnO catalyst was reduced in hydrogen for 3 h, the reduction temperature was 280 °C, and the...
Embodiment 3
[0044] (1) CuO / ZnO / Al 2 o 3 Catalyst preparation
[0045] Basic copper carbonate, basic zinc carbonate and aluminum oxide according to Cu 2+ :Zn 2+ :Al 3+ =58:25:17 Weigh it and add it to the ball mill tank 1, and add stainless steel balls according to the mass ratio of balls to materials at 20:1. Then add an appropriate amount of acetone to the ball milling tank to make the powder sample into a paste, load the ball milling tank on the ball mill, and ball mill at 200 rpm, 300 rpm, and 400 rpm for 20 h, stop every 2 h, Stop for 12 minutes each time. After ball milling, the steel balls were separated from the sample and the catalyst precursor was collected after the acetone volatilized. The obtained catalyst precursor was calcined at 320 °C for 3 h, and black CuO / ZnO / Al was obtained after the end 2 o 3 catalyst.
[0046] (2) CuO / ZnO / Al 2 o 3 Catalyst Characterization
[0047] CuO / ZnO / Al 2 o 3 The catalyst was reduced in hydrogen for 3 h, the reduction temperature w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com