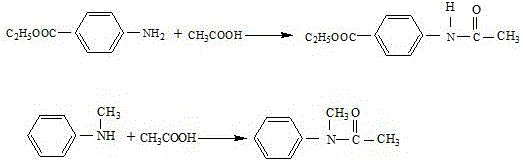
Preparation method of N,(4-ethyoxyl carbonyl phenyl)-N'-methyl-N'-phenyl formamidine
A technology of ethoxycarbonyl phenyl and phenylformamidine, which is applied in the field of fine chemical industry, can solve the problems of strong corrosion, long reaction process, complicated operation and the like, and achieves the effects of mild reaction conditions, convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
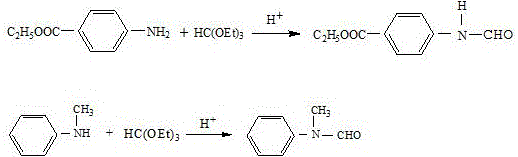
[0026] 165 grams (1mol) of ethyl p-aminobenzoate, 107 (1mol) of N-methylaniline, 106 grams (1mol) of trimethyl orthoformate and 5.1 grams (0.05mol) of a specific surface area of 137m 2 Put γ-type activated alumina per gram into a 500ml reaction bottle, stir and raise the temperature to 50°C~60°C, and keep it warm for 2h. Turn on the vacuum pump, gradually increase the vacuum degree, and distill the generated methanol under reduced pressure until no methanol is distilled.
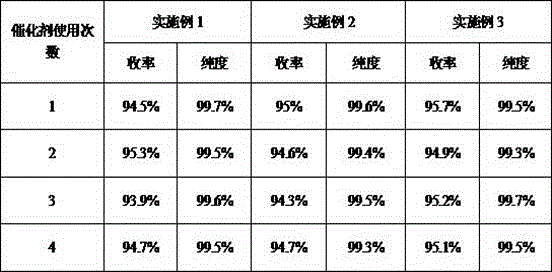
[0027] Filter the reaction solution while it is hot, reuse the recovered activated alumina, and distill the mother liquor under reduced pressure to obtain light yellow viscous liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl Formamidine 266.5 grams, yield 94.5%, purity 99.7%.
[0028] The recovered activated alumina was reused 3 times, and other preparation methods were the same as in Example 1. The results are shown in Table 1.
Embodiment 2
[0030] 165 grams (1mol) of ethyl p-aminobenzoate, 117.7 grams (1.1mol) of N-methylaniline, 177.8 grams (1.2mol) of triethyl orthoformate and 10.2 grams (0.1mol) of a specific surface area of 137m 2 Put γ-type activated alumina per gram into a 500ml reaction bottle, stir and raise the temperature to 50°C~60°C, and keep it warm for 2h. Turn on the vacuum pump, gradually increase the vacuum degree, and distill the generated ethanol under reduced pressure until no ethanol is distilled.
[0031] Filter the reaction solution while it is hot, reuse the recovered activated alumina, and distill the mother liquor under reduced pressure to obtain light yellow viscous liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl 268 grams of formamidine, yield 95%, purity 99.6%.
[0032] The recovered activated alumina was reused 3 times, and other preparation methods were the same as in Example 2. The results are shown in Table 1.
Embodiment 3
[0034] 165 grams (1mol) of ethyl p-aminobenzoate, 112.35 grams (1.05mol) of N-methylaniline, 159 grams (1.5mol) of trimethyl orthoformate and 10.2 grams (0.1mol) of a specific surface area of 137m 2 Put γ-type activated alumina per gram into a 500ml reaction bottle, stir and raise the temperature to 50°C~60°C, and keep it warm for 2h. Turn on the vacuum pump, gradually increase the vacuum degree, and distill the generated methanol under reduced pressure until no methanol is distilled.
[0035] Filter the reaction solution while it is hot, reuse the recovered activated alumina, and distill the mother liquor under reduced pressure to obtain light yellow viscous liquid N-(4-ethoxycarbonylphenyl)-N'-methyl-N'-phenyl Formamidine 270 grams, yield 95.7%, purity 99.5%.
[0036] The recovered activated alumina was reused 3 times, and other preparation methods were the same as in Example 3. The results are shown in Table 1.
[0037] Table 1 Catalyst recycling reaction results
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com