Application of compound gymnadenia conopsea pills in preparation of medicine for treating depression
A technology for depression and postpartum depression, which is applied in the field of medicine and can solve the problems of undiscovered reports or studies on the treatment of depression with Compound Shoushen Pills
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
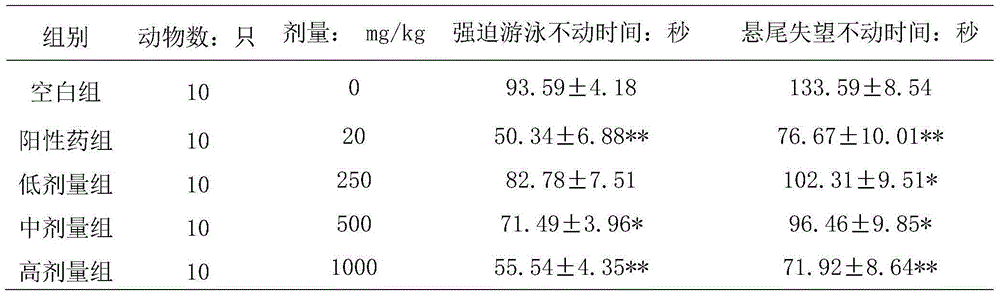
[0018] Experimental example 1. Improvement effect on forced swimming and tail suspension disappointed depression model mice
[0019] 1. Experiment preparation
[0020] Male Kunming mice, body weight (20±2) g, license number: SCXK (Lu) 2008-0002, provided by Shandong Lukang Pharmaceutical Co., Ltd. Compound Shoushen Pills (Jinhe Tibetan Medicine Co., Ltd., batch number 20140511). Positive control drug fluoxetine hydrochloride dispersible tablets (Eli Lilly Suzhou Pharmaceutical Co., Ltd., batch number: 2842A, specification: 20 mg). Electronic balance (AL104), Mettler-Toledo Instrument (Shanghai) Co., Ltd., rotary evaporator (RE-52C), Shanghai Qingpu Huxi Instrument Factory.
[0021] 2. Experimental method
[0022] 1. Grouping and dosage
[0023] The mice were randomly grouped according to body weight, 10 in every group, divided into blank group (given same volume of distilled water); positive drug group (fluoxetine hydrochloride group 20mg / kg); and 1000mg / kg administration...
experiment example 2
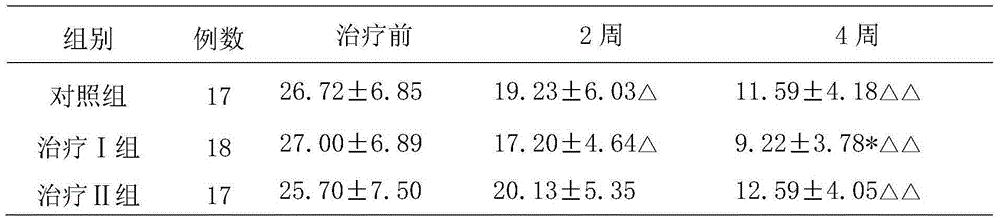
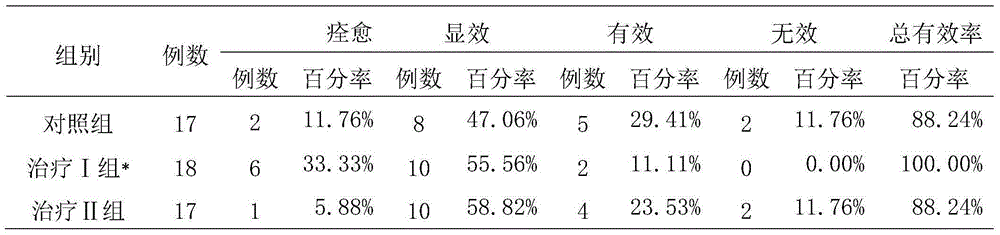
[0038] Experimental example 2. Clinical research on the treatment of postpartum depression with Fufang Shoushen Pills
[0039] 1. Patient data and diagnostic criteria
[0040] 1. General information:
[0041] 52 patients with postpartum depression, aged 23 to 48 years, with an average age of 33.1 years; 29 cases of cesarean section, 23 cases of natural delivery; 40 cases of primipara, 12 cases of multipara. They were randomly divided into treatment group Ⅰ (18 cases), treatment group Ⅱ (17 cases) and control group 17 cases. There was no significant difference (P>0.05) among the three groups in terms of age, mode of delivery, and number of deliveries (P>0.05), and they were comparable.
[0042] 2. Diagnostic criteria:
[0043] According to the diagnostic criteria in "Chinese Classification and Diagnostic Criteria for Mental Disorders" (CCMD-3) and "Diagnostic and Statistical Manual of Mental Disorders" (DSM-IV). Depression occurs within 2 weeks after delivery, and there are ...
experiment example 3
[0087] Experimental Example 3: Clinical Study of Compound Shoushen Pills in Treating Climacteric Depression
[0088] 1. Patient data and diagnostic criteria
[0089] 1. General information:
[0090] There were 49 menopausal depression patients, aged 52-68 years, with an average age of 59.1 years; 22 males and 27 females. They were randomly divided into a treatment group of 25 cases and a control group of 24 cases. There was no significant difference in age, sex, and HAMD score between the two groups (P>0.05), which were comparable.
[0091] 2. Diagnostic criteria:
[0092] According to the diagnostic criteria in "Chinese Classification and Diagnostic Criteria for Mental Disorders" (CCMD-3) and "Diagnostic and Statistical Manual of Mental Disorders" (DSM-IV). The course of disease lasts at least 2 weeks, and the HAMD-17 score is greater than 17 points. All patients excluded encephalopathy, no history of genetic psychiatry and acquired psychiatric history.
[0093] 2. Metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average age | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com