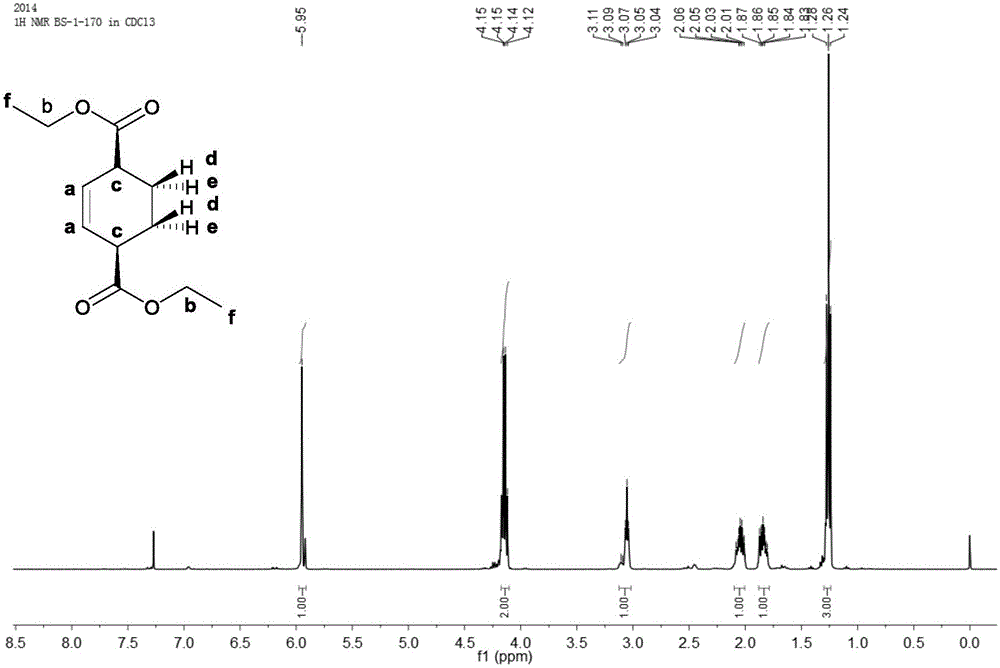
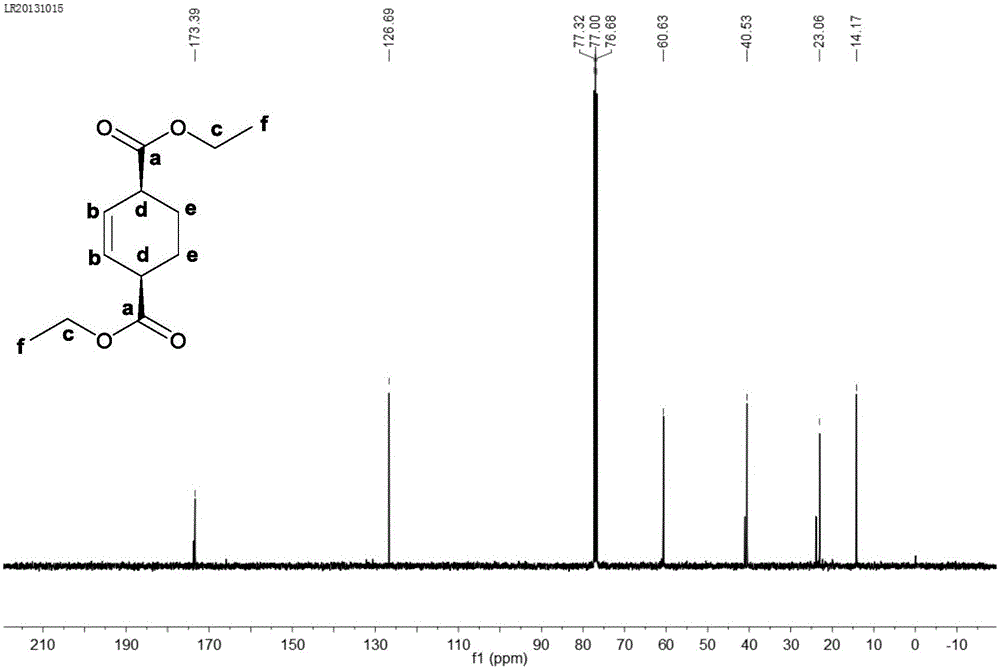
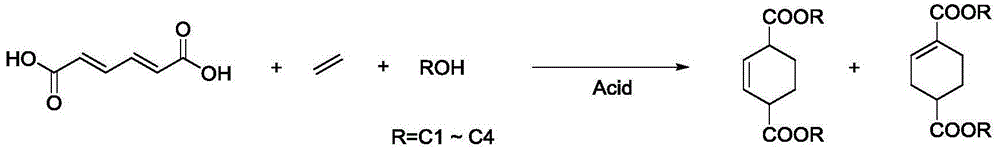
Preparation method of cyclohexene-1, 4-dicarboxylic acid diester
A technology of diformic acid diester and hexadienedioic acid, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of high cost and achieve the effect of reducing dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Trans, anti-hexadiene diacid esterification reaction and protonic acid catalyst performance evaluation of cycloaddition reaction
[0038] This example studies the performance of the homogeneous protonic acid catalyzed trans, trans-adixedienic acid in the esterification of ethanol and in the cycloaddition reaction with ethylene.
[0039]In the autoclave with polytetrafluoroethylene liner, add 1.58g trans, trans-hexadiene diacid and 15.8g ethanol, trans, the mass ratio of trans-hexadiene diacid and ethanol is 1:10, [H] The proton acid catalyst whose proton content is trans, trans-hexadienedioic acid molar weight 4%, after stirring and mixing uniformly at room temperature, feed ethylene of 1.0MPa, heat up to 180°C by electric heating, and magnetically stir at 1000rpm The reaction was carried out for 4 hours. Stirring was stopped, the reaction kettle was naturally cooled to room temperature, and ethylamine was neutralized to pH=7, and the product was qualitativel...
Embodiment 2
[0042] Example 2 Performance evaluation of solid acid catalysts for trans, trans-hexadiene diacid esterification and cycloaddition reactions performance of the reaction.
[0043] In the autoclave with polytetrafluoroethylene liner, add 1.58g trans, trans-hexadiene diacid and 15.8g ethanol, trans, the mass ratio of trans-hexadiene diacid and ethanol is 1:10, Trans, a solid acid catalyst with 10% trans-hexadiene dioic acid mass, stirred and mixed evenly at room temperature, passed 1.0 MPa of ethylene, heated to 180° C. by electric heating, and reacted for 4 hours under 1000 rpm magnetic stirring. Stirring was stopped, the reaction kettle was naturally cooled to room temperature, and ethylamine was neutralized to pH=7, and the product was qualitatively analyzed by gas chromatography-mass spectrometry, and high-performance liquid chromatography was used to determine trans, trans-hexadienedioic acid. The conversion rate was determined by gas chromatography to quantitatively determ...
Embodiment 3
[0048] Example 3 The amount of protonic acid on trans, the impact of trans-hexadiene diacid esterification and cycloaddition reaction
[0049] In this example, the effect of the amount of silicotungstic heteropolyacid on the esterification of trans-hexadieneddioic acid in ethanol and the cycloaddition reaction with ethylene was studied. It is specifically manifested in trans, trans-hexadiene diacid conversion rate and trans, trans-hexadiene diacid diester, 2-cyclohexene-1,4-dicarboxylate, 1-cyclohexene-1, On the selectivity of 4-dicarboxylic acid diester.
[0050] Add a certain amount of silicotungstic heteropolyacid catalyst into a high-pressure reactor with a polytetrafluoroethylene lining, and other conditions are the same as in Example 1, and perform performance evaluation according to the method in Example 1 of this patent. Table 3 shows the effect of the dosage of heteropoly acid of silicotungstic acid on the esterification of trans-hexadieneddioic acid with ethanol and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com