Preparation and Quality Standard Detection Method of Cordyceps Qishen Capsules
A technology of Cordyceps and capsules, which is applied in the field of preparation and quality standard detection of Cordyceps Qishen Capsules, can solve the problems of easy rejection, loss of active ingredients, single preparation method, etc., achieve simple and feasible preparation method, avoid the loss of ingredients, good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation method of Cordyceps Qishen Capsules comprises the steps of:
[0022] 1) Take 140g of Cordyceps sinensis, crush it into fine powder of Cordyceps sinensis;
[0023] 2) Take 1400g of astragalus, crush it, pass through a 200-mesh sieve, put it in a container, add twice the weight of 85% (v / v) ethanol, stir and extract at 200rpm, control the microwave power to 600W during the extraction process, and the extraction time is 50min Then place it at 4°C for 12 hours, filter, and filter the residue for later use; the filtrate is evaporated under reduced pressure to recover ethanol, and the filtrate is concentrated to extract A with a density of 1.2g / ml; the content of astragaloside IV is 1.27mg by high performance liquid chromatography / ml;
[0024] Add twice the weight of water to the filter residue, stir well, then add 0.5wt% neutral protease (200,000 U / g), enzymatically hydrolyze at 37°C for 120min, then boil for 5min, then add anhydrous Ethanol, stirred at 30...
Embodiment 2
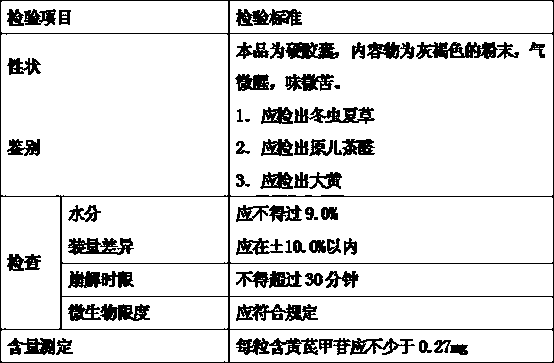
[0038] Cordyceps Qishen Capsule GPM workshop prescription and quality standard test of the present invention are shown in Table 1-2:
[0039] Table 1
[0040] name Dosage kg storage requirements Cordyceps sinensis 67.2 Store in a cool and dry place, away from moths Astragalus 672 Store in a ventilated and dry place, moisture-proof, moth-proof Danshen 268.8 keep in a dry place safflower 268.8 Store in a cool dry place, moisture-proof, moth-proof Suanzaoren 134.4 Store in a cool and dry place, away from moths starch Appropriate amount Keep tightly closed and store in a dry place. empty capsule 480,000 tablets Sealed and stored at a temperature of 10-25°C and a relative humidity of 35-65%.
[0041] Table 2
[0042]
Embodiment 3
[0044] Animal Toxicity Test
[0045] 40 healthy Kunming strain mice, half male and half female, body weight 18.3±1.9g, 40 mice were randomly divided into two groups, each group was half male and half male, of which 20 were the control group and fed with normal water; the other 20 Only mouse is given the capsule preparation prepared by embodiment 1, dosage is 200mg / kg, three times every day, application mouse carries out toxicity experiment and shows: compare with matched group, after administration, mouse does not see significant difference, experiment observes continuously for two weeks, The general condition, food intake, drinking water and weight gain of the mice were normal. On the day of administration and within two weeks after administration, no animal died, suggesting that the drug has low toxicity and is safe for clinical use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com