3-Hydroxy-2-naphthoic acid/1-octanesulfonic acid/lyh complex and its synthesis method
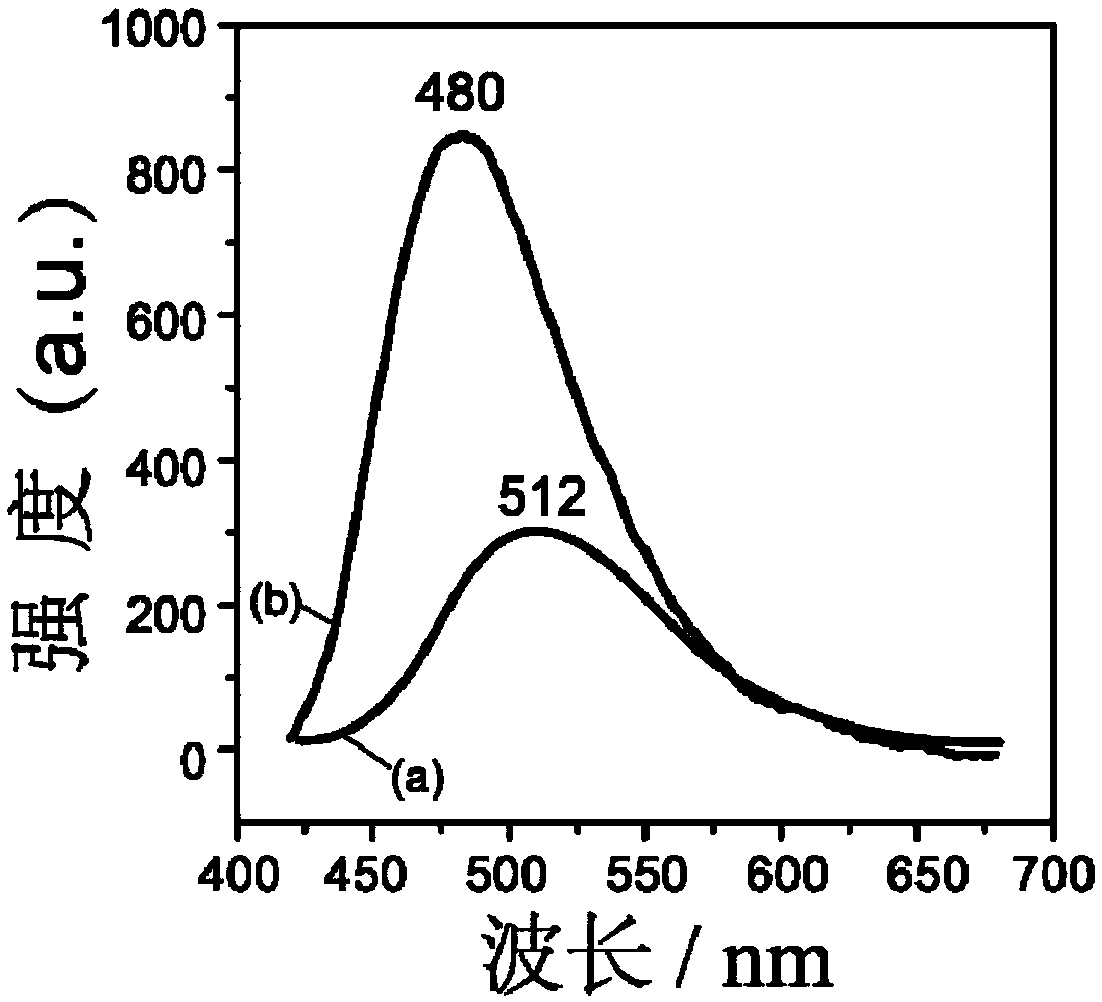
A synthesis method and octanesulfonic acid technology are applied in the field of 3-hydroxy-2-naphthoic acid/1-octanesulfonic acid/LYH complex and its synthesis, and can solve the problem of fluorescence of 3-hydroxy-2-naphthoic acid sodium salt Low emission intensity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention also provides a synthesis method of the above-mentioned complex, which may include the following steps:
[0034] a) With sodium hydroxide or potassium hydroxide as alkaline reagent, 1-octane sodium sulfonate, alkaline reagent and 3-hydroxyl-2-naphthoic acid are dissolved in water to obtain a mixed solution, wherein, 3-hydroxyl-2- The molar ratio of naphthoic acid and alkaline reagent is 1: (1-2); the molar ratio of 3-hydroxy-2-naphthoic acid and 1-octane sodium sulfonate is 1: (3-5);
[0035] Since the LYH layer is positively charged, there are exchangeable anions between the layers, which are easy to combine with anionic compounds. Therefore, it is necessary to deprotonate 3-hydroxy-2-naphthoic acid to form an anionic compound so that it can be inserted into the LYH layer between. Specifically, sodium hydroxide or potassium hydroxide can be used as an alkaline reagent to react with 3-hydroxyl-2-naphthoic acid, thereby deprotonating 3-hydroxyl-2-n...
Embodiment 1
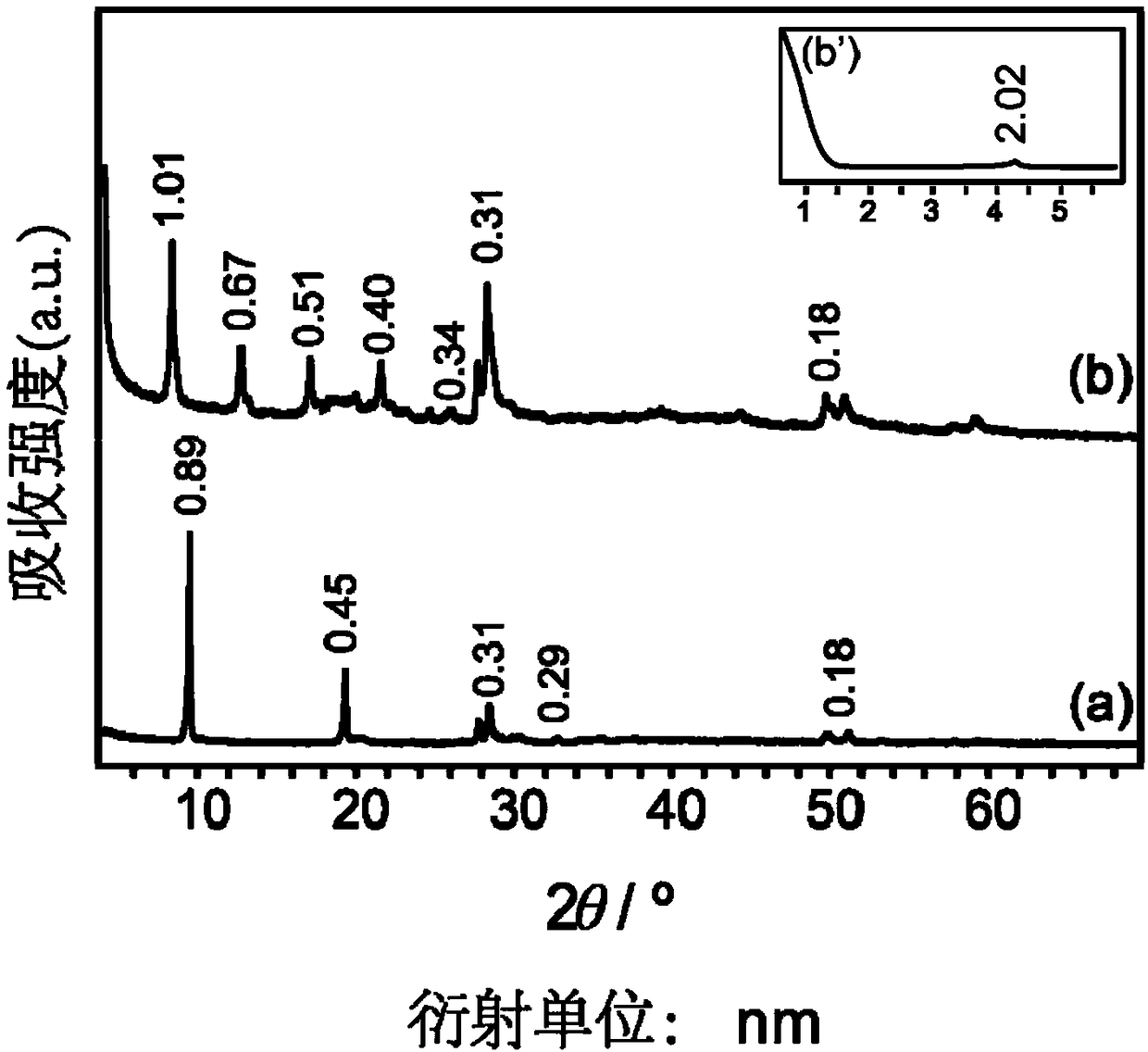
[0042] Example 1NO 3 Synthesis and structure characterization of -LYH
[0043] Synthesized by homogeneous precipitation method, specifically as follows: 0.3383g (1mmol) Y(NO 3 ) 3 ·6H 2 O, 1.10g (13mmol) NaNO 3 , 0.14g (1mmol) hexamethylenetetramine (HMT) was dissolved in 80mL exhaust water, transferred to the reactor and passed N 2 5 minutes, then hydrothermal reaction at 90°C for 12 hours; after the reaction, the product obtained was filtered with suction, washed with deionized water and then filtered with suction, repeated 3 times, and dried in vacuum for 24 hours to obtain white powder NO 3 -LYH 0.177g. With Y(NO 3 ) 3 ·6H 2 Based on O, the yield was 96.2%.
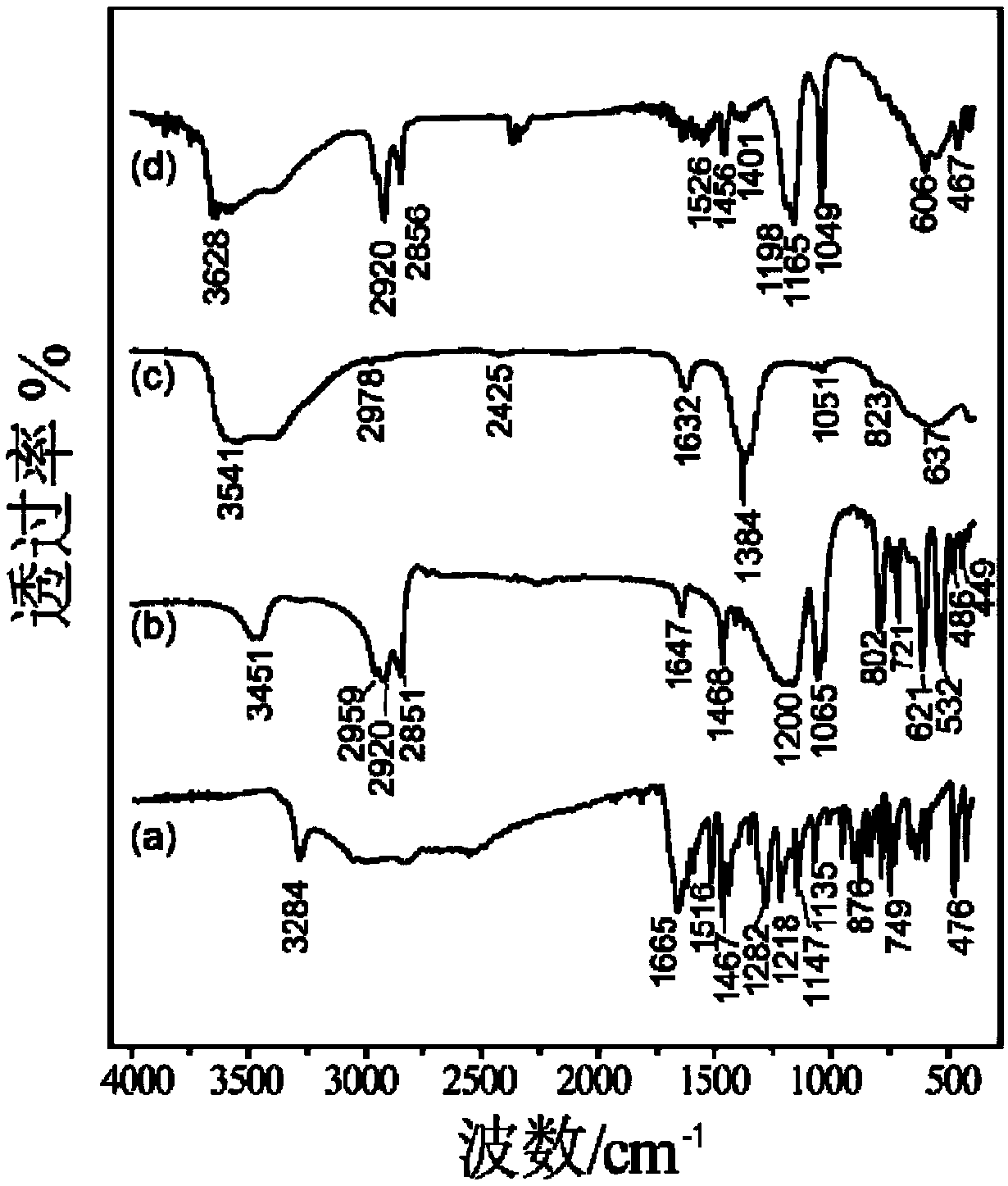
[0044] The elemental analyzer (model: Vario EL) produced by German Elementar Company was adopted to measure the NO synthesized in the embodiment of the present invention 1. 3 -C, H, N content of LYH; Adopt the plasma inductively coupled atomic emission spectrometer (ICP) (model: SPECTROARCOSEOP) that German ...
Embodiment 2
[0048] Synthesis and structural characterization of embodiment 2 complex
[0049] 0.0607g (0.3225mmol) of 3-hydroxy-2-naphthoic acid, 0.0129g (0.3225mmol) of sodium hydroxide and 0.2267g (0.9675mmol) of sodium 1-octanesulfonate were added to 8ml of deionized water, Ultrasound dissolves 3-hydroxy-2-naphthoic acid, sodium hydroxide and sodium 1-octanesulfonate to obtain a mixed solution containing sodium 3-hydroxy-2-naphthoate and sodium 1-octanesulfonate;
[0050] 0.07912g (0.43mmol) of NO 3 -LYH was dispersed in 130ml of deionized water, added to the mixed solution, mixed evenly, transferred to a reaction kettle, and hydrothermally reacted at 70°C for 24 hours. After the reaction, filter under reduced pressure, then wash the product with deionized water, repeat 3 times, and then dry it in vacuum at 40°C for 24 hours to obtain 3-hydroxy-2-naphthoic acid / 1-octanesulfonic acid / LYH complex 0.1011g, with NO 3 -LYH as the benchmark, the yield was 91.4%.
[0051] Elemental analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com