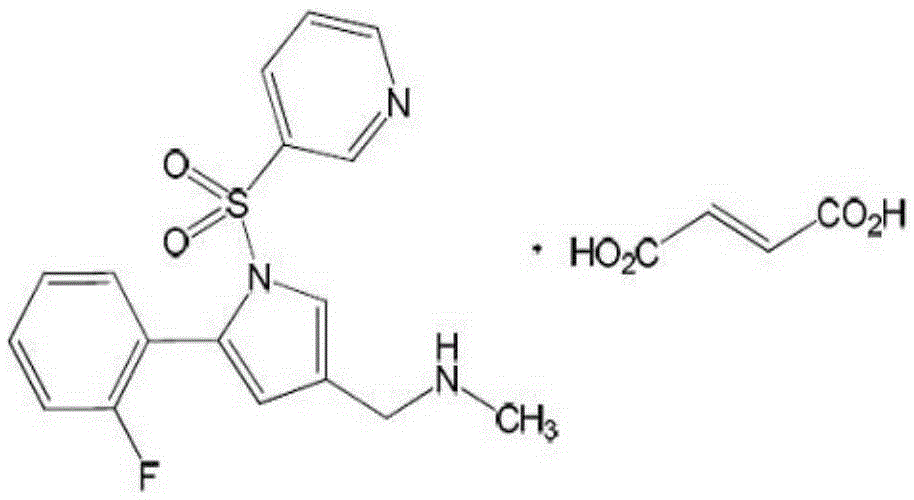
Novel application of gastric acid secretion inhibitor
A technology for gastric mucosa and lonapridine fumarate, which is applied to medical preparations containing active ingredients, organic active ingredients, and the digestive system, and can solve problems such as the treatment and prevention of undisclosed lonapridine fumarate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
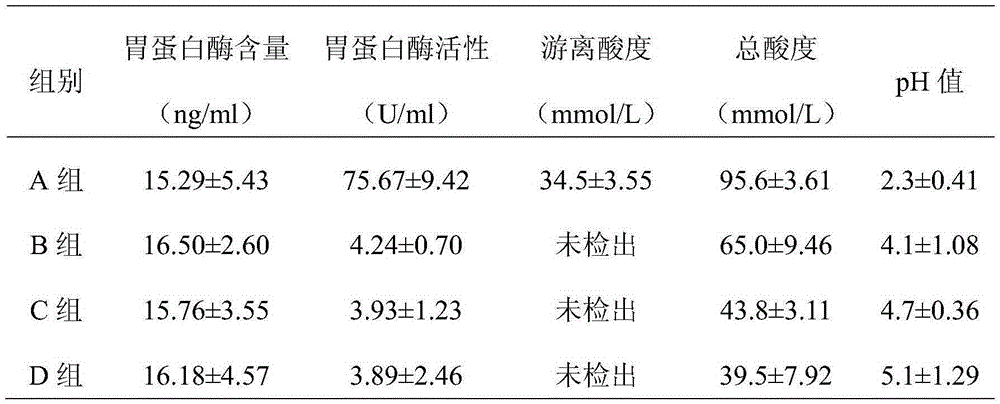
Image
Examples
Embodiment 1
[0104] Preparation of Lonapridine Fumarate Small Needle Injection:
[0105] Specification: 5ml: 10mg
[0106] 1. Prescription:
[0107]
[0108] Second, the preparation process:
[0109] 1. Weighing and dosing:
[0110] Weigh the prescribed amount of glucose and edetate calcium disodium, add it to the water for injection with 80% of the total amount prepared (water temperature <50°C), stir and dissolve; then weigh the prescribed amount of lonapridine and add it to the liquid preparation tank Stir well until completely dissolved. Then add 0.1% (g / ml) activated carbon for needles, stir evenly, let stand for adsorption for 15 minutes, and filter the medicinal solution through a 0.45 μm filter to remove carbon.
[0111] 2. pH adjustment:
[0112] Adjust the pH value to 2.5-3.5 with 0.1mol / L hydrochloric acid solution, add water for injection to the full amount, and filter the medicinal solution through a 0.22 μm microporous filter.
[0113] 3. Filling and sealing
[0114...
Embodiment 2
[0119] Preparation of Lonapridine Fumarate Injection:
[0120] Specifications: 20ml: 20mg
[0121] 1. Prescription:
[0122]
[0123] Second, the preparation process:
[0124] 1. Weighing and dosing:
[0125] Weigh the prescribed amount of glucose and edetate calcium disodium, add it to the water for injection with 80% of the total amount prepared (water temperature <50°C), stir and dissolve; then weigh the prescribed amount of lonapridine and add it to the liquid preparation tank Stir well until completely dissolved. Then add 0.1% (g / ml) activated carbon for needles, stir evenly, let stand for adsorption for 15 minutes, and filter the medicinal solution through a 0.45 μm filter to remove carbon.
[0126] 2. pH adjustment:
[0127] Adjust the pH value to 2.5-3.5 with 0.1mol / L hydrochloric acid solution, add water for injection to the full amount, and filter the medicinal solution through a 0.22 μm microporous filter.
[0128] 3. Filling and sealing
[0129] After pas...
Embodiment 3
[0134] Preparation of Lonapridine Fumarate Large Infusion Injection:
[0135] Specification: 100ml: 20mg
[0136] 1. Prescription:
[0137]
[0138] Second, the preparation process:
[0139] 1. Weighing and dosing:
[0140] Weigh the prescribed amount of glucose and edetate calcium disodium, add it to the water for injection with 80% of the total amount prepared (water temperature <50°C), stir and dissolve; then weigh the prescribed amount of lonapridine and add it to the liquid preparation tank Stir well until completely dissolved. Then add 0.1% (g / ml) activated carbon for needles, stir evenly, let stand for adsorption for 15 minutes, and filter the medicinal solution through a 0.45 μm filter to remove carbon.
[0141] 2. pH adjustment:
[0142]Adjust the pH value to 5.0-6.5 with 0.1mol / L hydrochloric acid or 0.1mol / L sodium hydroxide solution, add water for injection to the full amount, and filter the drug solution through a 0.22μm microporous filter.
[0143] 3. Pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com