Preparation method of hyperbranched poly (urethane-amine) with hydroxide radical serving as end group and internal branched units provided with vinyl
A branched unit and hyperbranched polymerization technology, applied in the field of preparation of hyperbranched poly(urethane-amine), can solve problems such as single functional group structure, and achieve the effects of simple synthesis method, high yield and easy availability of synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
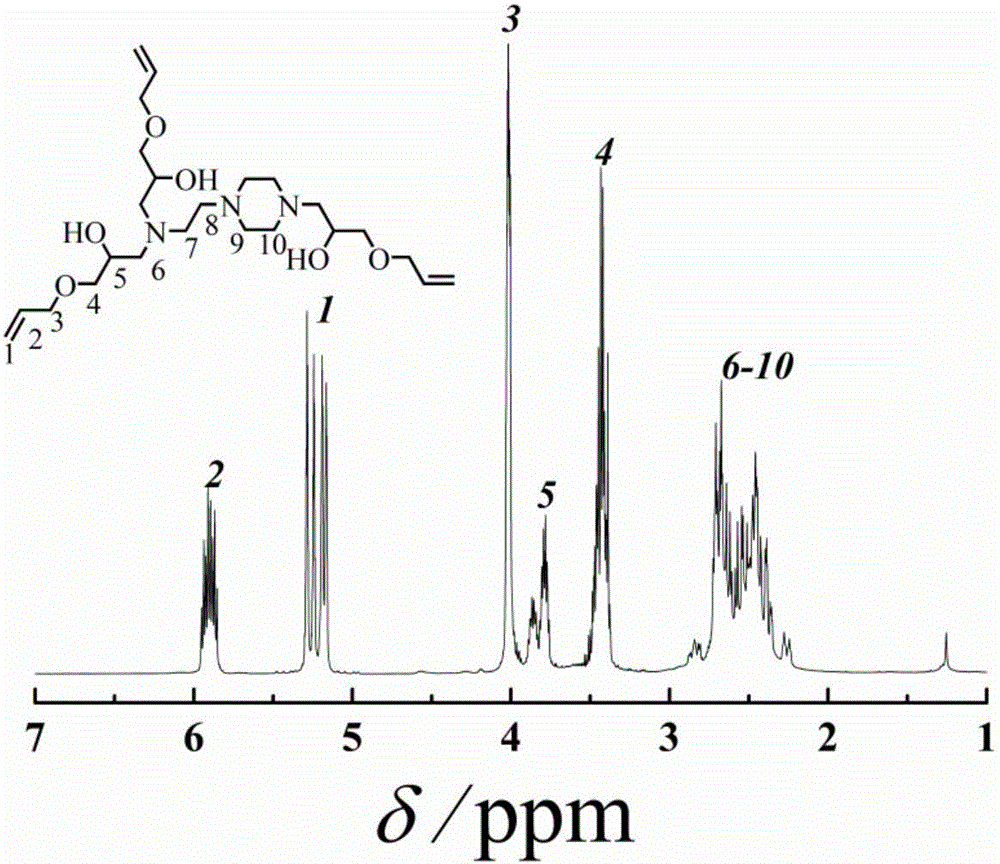
[0021] Add N-aminoethylpiperazine (2.96g, 22.9mmol), anhydrous methanol (11mL) into the reactor, stir and pass N 2 Remove oxygen for 20-30 minutes, and after it is fully dissolved, add allyl glycidyl ether (7.83g, 68.6mmol) strictly according to the ratio of N-aminoethylpiperazine to allyl glycidyl ether molar ratio of 1:3 mixture, in N 2 Under ambient conditions, react in an oil bath at 60°C for 10 hours, and finally become a colorless transparent liquid. The obtained solution was rotary evaporated at 30° C. to remove the solvent anhydrous methanol, and then dried in vacuum at room temperature for 2 h, and the obtained product was light yellow oily slightly viscous liquid.
Embodiment example 2
[0023] Add 2-aminomethylpiperidine (2.62g, 22.9mmol), absolute ethanol (10mL) into the reactor, stir and pass N 2 Remove oxygen for 20-30 minutes, and after it is fully dissolved, add allyl glycidyl ether (7.83g, 68.6mmol) strictly according to the ratio of N-aminoethylpiperazine to allyl glycidyl ether molar ratio of 1:3 mixture, in N 2 The reaction was carried out in an oil bath at 60°C for 10 hours, and finally a colorless transparent liquid was obtained. The obtained solution was rotary evaporated at 30°C to remove the solvent anhydrous methanol, and then dried in vacuum at room temperature for 2 hours. The obtained product was a light yellow oily slightly viscous liquid.
Embodiment example 3
[0025] Add 4-aminopiperidine (2.29g, 22.9mmol), anhydrous methanol (11mL) into the reactor, stir and pass N 2 Remove oxygen for 20-30 minutes, and after it is fully dissolved, add allyl glycidyl ether (7.83g, 68.6mmol) into the mixture strictly according to the molar ratio of 4-aminopiperidine to allyl glycidyl ether: 1:3 , at N 2 The reaction was carried out in an oil bath at 60°C for 10 hours, and finally a colorless transparent liquid was obtained. The obtained solution was rotary evaporated at 30°C to remove the solvent anhydrous methanol, and then dried in vacuum at room temperature for 2 hours. The obtained product was a light yellow oily slightly viscous liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com