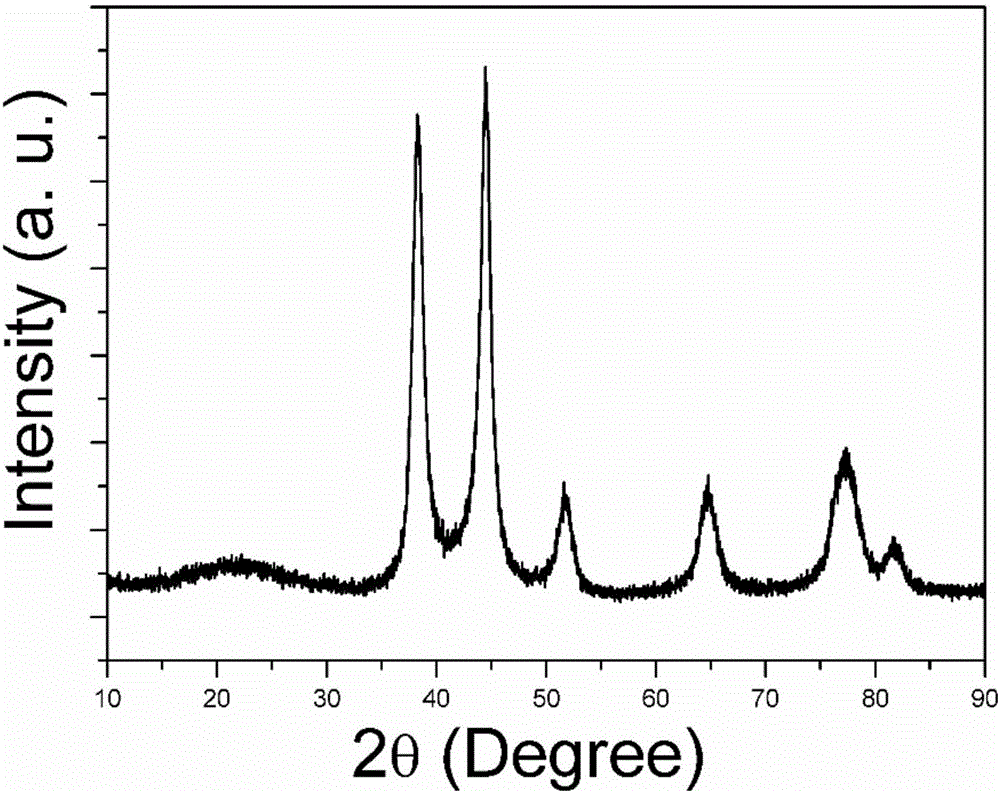
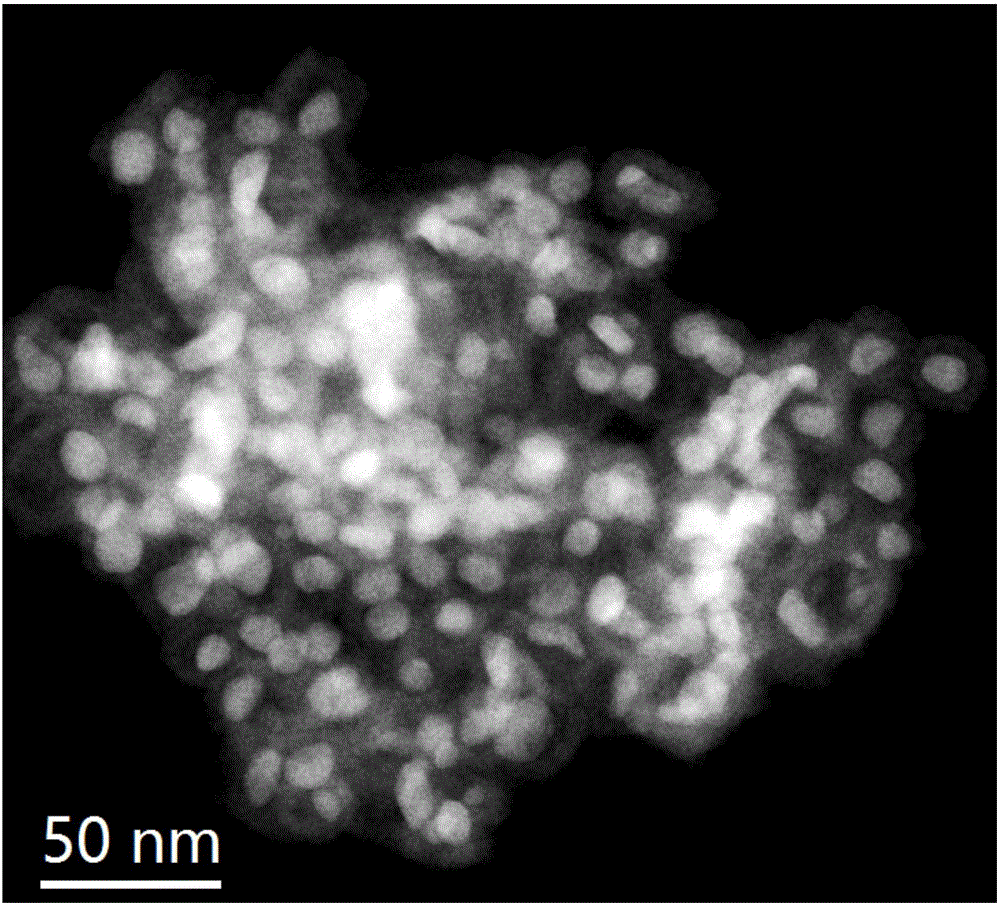
Preparation method for NiAu nano catalyst used for ammonia borane hydrogen-production
A nano-catalyst and borane-ammonia technology is applied in the field of preparation of NiAu binary nanoparticles, which can solve the problems of difficulty in wide application, easy poisoning of platinum group elements, and decreased catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0022] Step 1: Heat 10 ml of oleylamine solution to 120°C, quickly pour 1.68 mmol of nickel nitrate hexahydrate and 1 ml of ethanol solution dissolved with 0.12 mmol of chloroauric acid tetrahydrate into the above oleylamine solution and maintain magnetic stirring for 20 minutes , during which the solution boils and a large number of bubbles emerge from the surface. During the heating process, the solution changed from green to purple-black. When the temperature rises to about 220°C, the solution starts to bump and boil, and evaporating products are released appropriately, making the system balanced and stable. When the temperature reached 240° C., the solution turned black rapidly. After maintaining for 10 minutes, the heating was stopped and cooled to room temperature naturally.
[0023] Step 2: Pour 40 ml of isopropanol into the reaction solution, stir thoroughly, and wash repeatedly 3 times by ultrasonication and centrifugation. Then use a 1:1 volume ratio of n-hexane an...
Embodiment approach 2
[0028] The difference between this embodiment and Embodiment 1 is that in step 1, 10 ml of oleylamine is added at room temperature with 0.04 mmol of nickel salt and 1 ml of ethanol solution in which 0.03 mmol of chloroauric acid is dissolved.
Embodiment approach 3
[0030] The difference between this embodiment and Embodiment 1 is that the highest temperature in step 1 is set to 300° C., and the holding time is set to 5 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com